It is commonly and erroneously believed that more than six minutes of no heartbeat results in immediate destruction of brain capacity. Some scientists who criticize cryonics are concerned about postmortem degradation of brain structure. One physician and scientist who was interested in cryonics expressed the opinion that he would not want to be cryopreserved if the ischemic period was 2 hours or more because he believed critical brain structure would be degraded. (Ischemia is the condition of lack of oxygen & nutrient delivered to body tissues due to a lack of blood flow.) Are these experts correct? If two hours is not a correct evaluation of the critical time period, then when does postmortem degradation of brain structure become so extensive that no potential future molecular repair technology has a possibility of repairing it -- ie, so extensive that cryonics has no possible value. This analysis of peer-reviewed literature will attempt to answer that question.
It would be helpful to have reference to potassium-sodium ratios, cellular ATP content, electron micrograph data, MRI scans and blood levels of Lactic DeHydrogenase (LDH) to quantify ischemic damage, but the literature has little such data available that would be useful for evaluation for cryonics purposes.

|
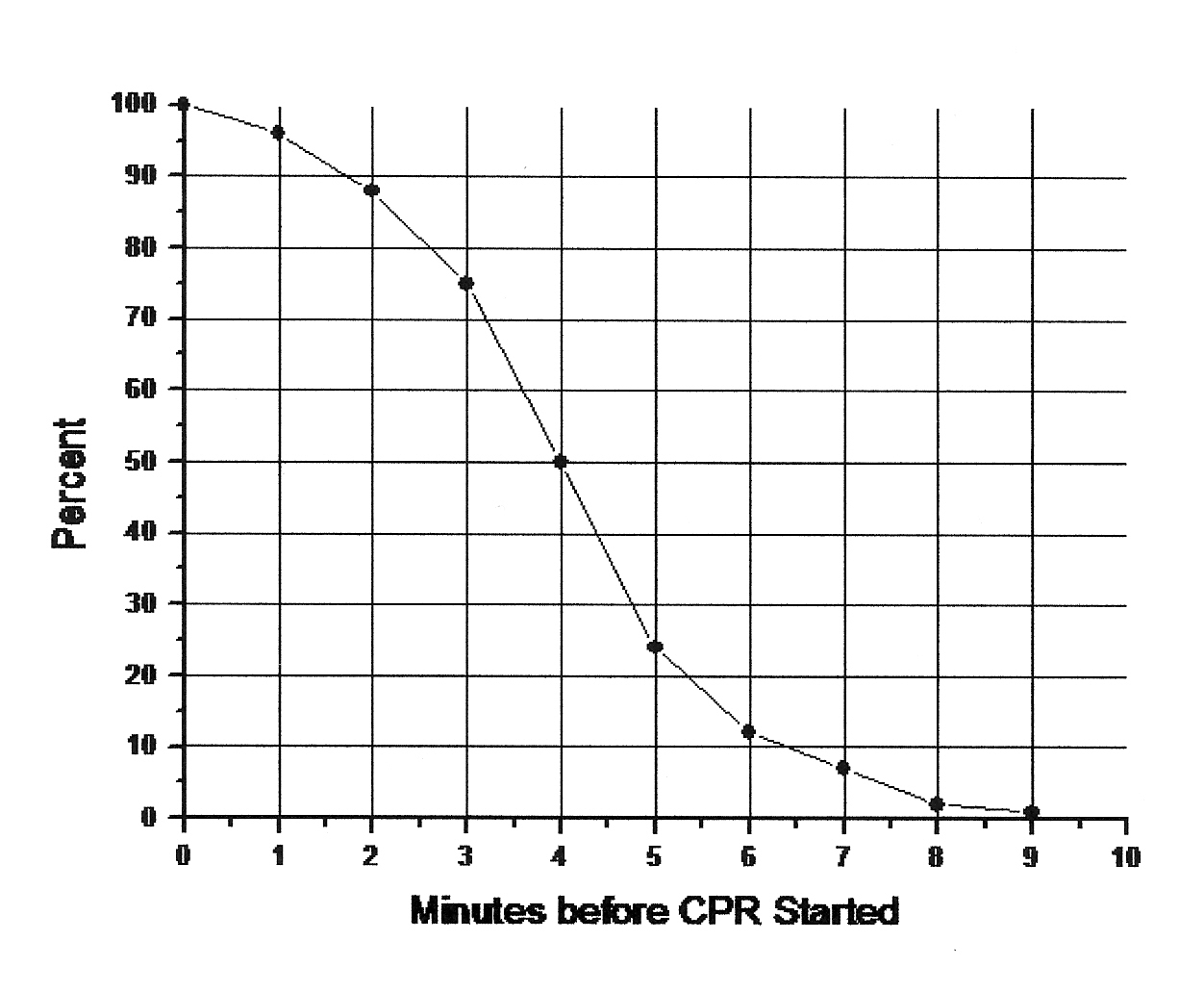
A person who suffers cardiac arrest (ie, the heart stops beating) and survives usually has brain damage if circulation is not restarted within about five minutes. Experimental animals have survived longer periods without blood circulation at room temperature without suffering brain damage [ANNALS OF EMERGENCY MEDICINE; Safar,P; 22(2 Pt 2):324-349 (1993)], but healthy laboratory animals are usually more hardy than the typical human cardiac arrest victim (or cryonics patient).
An experiment with rats in which the middle cerebral artery was occluded found few signs of neuron necrosis (death) after 4 hours. After 6 hours only 15% of the neurons were necrotic. Only by 12 hours were 65% of the neurons necrotic [STROKE; Garcia,JD; 26(4):636-643 (1995)]. Similar results had been found in experiments performed more than a decade earlier [ANNALS OF NEUROLOGY; Pulsinelli,WA; 11:491-498 (1982)]. In the brains of rats euthanized at room temperature, only after 9 hours did neurons show the first signs of autolysis: disappearance of ribosomes and a marked increase (to 2.5%) of cortical neurons strongly-staining for caspase−3 [INTERNATIONAL JOURNAL OF CLINICAL AND EXPERIMENTAL PATHOLOGY; Sheleg,SV; 1(3):291-299 (2008)]. Rat brain neurons in cortical slices recover function upon reoxygenation as well after five hours post-mortem as they do after immediate post-mortem reoxygenation [PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES (USA); Charpak,S; 95(8):4748-4753 (1998)].
Electron microscopy of cat brains subjected to 2 hours of no blood flow showed no evidence of lysosome breakage [VIRCHOWS ARCHIV B; Kalimo,H; 25:207-220 (1977)]. Assays of neurons extracted an average of 2.6 hours from elderly humans post-mortem showed 70 to 90% of the neurons to be viable after 2 weeks in vitro, by various measures of viability [AMERICAN JOURNAL OF PATHOLOGY; Konishi,Y; 161(5):1567-1576 (2002)]. An experiment on rats subjected to 5−6 hours of cardiac arrest showed that upon reperfusion reactivated brain tissue showed normal synaptic activity [PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES (USA); Charpak,S; 98(8):4748-4753 (1998)]. A study of hippocampal slice tissue (the brain tissue most vulnerable to ischemic injury) extracted up to 3 hours post-mortem showed the retention of significant spiking capability [EXPERIMENTAL NEUROLOGY; Leonard,BW; 113(3):373-377 (1991)]. The spin-trapping agent NXY-059 given 15 minutes after rats had been subjected to 2 hours of ischemia reduced infarct volume and neurological impairment by 59% (indicative of preservation of functional capacity and functional recoverability -- without even the need of repair -- in a time period greater than 2 hours) [BRITISH JOURNAL OF PHARMACOLOGY; Sydserff,SG; 135(1):103-112 (2002)].
How can these results be reconciled with the "5 minute limit" for cardiac arrest victims to survive without brain damage?
The answer is that although a few minutes of ischemia is enough to put brain tissue cells onto an irreversible path of self-destruction, it takes many hours for the destructive process to actually occur. The rate at which that damage occurs is greatly worsened by the reperfusion injury (damage resulting from restarting blood circulation, mainly due to free radicals) inflicted by re-starting circulation after too many minutes of ischemia. And reperfusion becomes increasingly difficult with the passage of ischemic time -- the "no−reflow" phenomenon.
An ultrastructure study of rat neurons showed that after 3 hours of brain tissue ischemia cytoplasm was only mildly swollen and the mitochondria showed only moderate swelling, with some disruption of inner mitochondrial membrane. After 5 hours of ischemia neuronal cytoplasmic swelling was severe and mitochondria were moderately to severely swollen. After 24 hours the neuron mitochondria, although severely disrupted, were nonetheless distinguishable. By contrast, after 3 hours of ischemia followed by 2 hours of reperfusion cytoplasm was severely swollen and mitochondria showed poor structural integrity. When reperfusion was continued for 24 hours mitochondria were degenerating and there was a complete loss of the mitochondrial outer membrane shape and form [STROKE; Solenski,NJ; 33(3):816-824 (2002)].
Dogs subjected to cardiac arrest times of greater than 10 minutes show considerable brain damage, particularly in the CA1 region of the hippocampus when evaluated after 96 hours [STROKE; Radovsky,A; 26(11):2127-2134 (1995)]. Rats subjected to 15 minutes of ischemia showed increased expression of pro-apoptotic ("cell suicide") caspase−3 enzymes 8 hours after reperfusion in many brain areas (up to 72 hours in the CA1 region of the hippocampus. Between 8−24 hours caspase−3−like activity is elevated about ten-fold in the hippocampus [THE JOURNAL OF NEUROSCIENCE; Chen,J; 18(12):4914-4928 (1998)]. Rats subjected to 3 hours of hypoxia (no oxygen) show 80% cell death by 24 hours, most of which is by necrosis (cell killing) rather than apoptosis (cell suicide) [CELL RESEARCH; Jones,PA; 14(3):241-250 (2004)].
Unpublished data from Cryonics Institute cryobiologist Dr. Yuri Pichugin indicates that by 24 hours of warm ischemia hippocampal tissue has K/Na ratios that are about half normal, and that by 48 hours are at the level of complete non-viability. What this means in terms of neuron survival is debatable, partly because the assay does not distinguish neurons from glial cells. From a cryonics point of view, even a 20% viability could be encouraging. The fact that the cells are only maintaining 20% of the normal Potassium/Sodium ion ratios across cell membranes indicates a low energy status of cells, but it also indicates that cell membranes are at least enough intact to maintain some ion gradient -- ie, cell structure has not been damaged.
These data suggest that for those who believe that freezing damage can someday be repaired by nanotechnology, a cryonics patient could tolerate at least 5 hours of ischemia at room temperature before being straight-frozen -- while preserving most memory and identity.
|
Resistance to blood flow increases after six minutes of cardiac arrest. Prior to six minutes of ischemia, a cerebral perfusion pressure of 25 mmHg (typical of the pressure achieved by CPR -- CardioPulmonary Resuscitation) is adequate to restore cerebral blood flow. After 6 minutes the cerebral perfusion pressure must be 35 mmHg and beyond 12 minutes even 35 mmHg cannot restore cerebral blood flow [CRITICAL CARE MEDICINE; Shaffner,DH; 27(7):1335-1342 (1999)]. After 30 minutes of ischemia, cats require 100 mmHg of pressure to prevent no-reflow [INTENSIVE CARE MEDICINE; Iijima,T; 19:82-88 (1993)].
It was for this reason that Peter Safar, the "father of CPR", advocated wide distribution of spring-loaded pens for rapid intra-muscular epinephrine injection (epi−pens) as a lifesaving adjunct to CPR. His words have not been heeded by many authorities and epinephrine remains unavailable without prescription in the United States and the United Kingdom. True enough, epineprine can be dangerous if an epi−pen injects into a vein (the intra-muscular dose is ten times what would be injected into the circulatory system during ACLS, Advanced Cardiac Life Support), so it is hard to say how many more are lives lost by lack of availability for bystander CPR than are saved by prevention of misuse. However, epinephrine is available without prescription in Canada and can be ordered from Canada in the United States. The easy-to-use epi−pen that can inject epinephrine into the thigh (counteracting the sometimes fatal body-wide vasodilation and loss of blood pressure that can occur within minutes of anaphylaxis) can be ordered from Canadadrugsuperstore.com and will not be seized by US Customs. These devices should be included in cryonics emergency "first aid" kits. ("Twinject" devices might also be convenient.) Used in conjunction with cardiopulmonary support of a cryonics patient, epinephrine can greatly assist in improving blood flow.
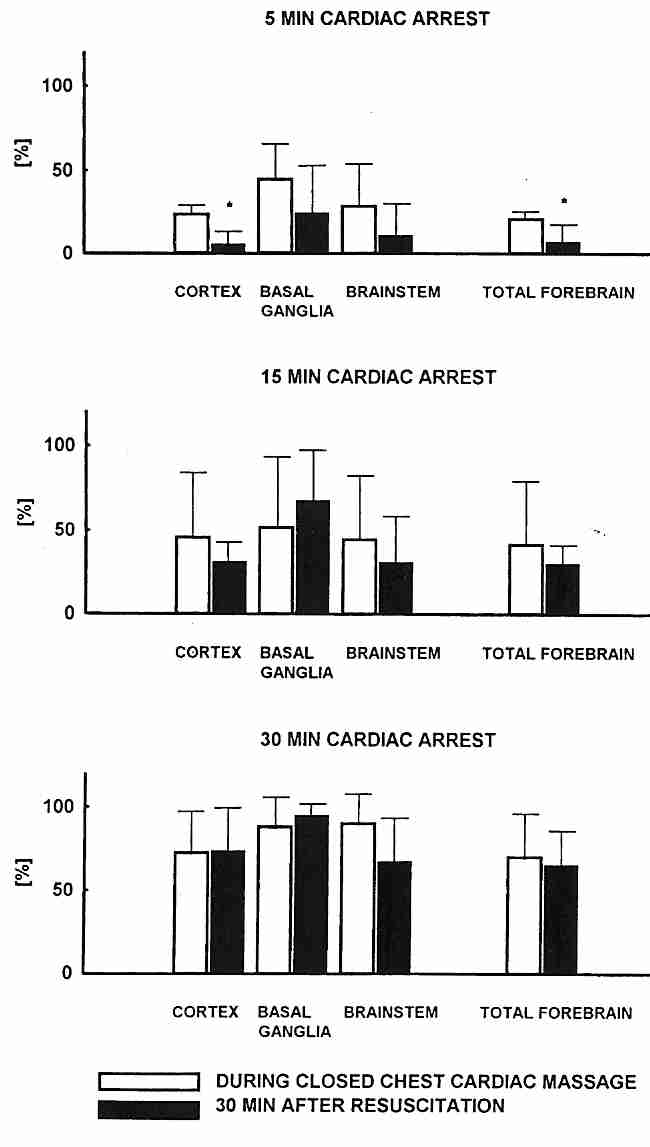
The "no−reflow" problem affects blood vessels in some regions of the brain more than others. In cats, the ability to reperfuse the basal ganglia is particularly difficult after 15−30 minutes of ischemia. In fact, 30 minutes after the heart has been re-started (following ischemia) the no−reflow is even worse than before the heart re-started, indicative of reperfusion injury [INTENSIVE CARE MEDICINE; Fischer,M; 21:132-141 (1995)].
Another means of overcoming "no−reflow" is by reducing
hematocrit, ie, reducing the number of red blood cells in
circulation. When blood has been static (not circulating) for a period of time,
the blood cells aggregate and impede blood flow. Normally red blood cells
account for just under half of blood volume (hematocrit of just under 50%),
but hematocrit can be reduced to 25% in healthy dogs without compromise in oxygen
delivery [STROKE; Leonov,Y; 23(1):45-53 (1992)]. Hematocrit, however, can
be regarded as a rough index for the quantity of hemodilution, because dilution of
fibrinogen and other blood substances that can contribute to "no-reflow"
affect the vascular resistance. Hemodilution has often
been achieved by replacing some of the blood with dextran 40 (a glucose
polymer with molecular weight of 40,000 daltons), which maintains osmotic pressure and
prevents edema. (For more details on the perfusion mechanisms, see my essay
Perfusion & Diffusion in Cryonics
Protocol.)
|
Heparin has been used by the Cryonics Institute to prevent blood clotting in cryonics patients prior to shipment to CI by a funeral director. Heparin has anti-inflammatory and anti-histaminic properties along with anti-clotting properties that may reduce edema along with the anti-coagulant effect [CURRENT RESEARCHES; Stullken,EH; 55(5):683-687 (1976) and JOURNAL OF CLINICAL INVESTIGATION; Wang,L; 110(1):127-136 (2002)]. To be effective, heparin should be given within 7 minutes of cardiac arrest. But with experimental ischemia in rabbits, cerebral impairment of blood circulation if heparin is given 15 minutes prior to 15 minutes of ischemia is not better than if heparin is not given [STROKE; Fischer,EG; 3(5):538-542 (1972)], but hemodilution with saline prior to ischemia vitually eliminates vessel occlusion. (This experiment established that it is blood aggregation rather than narrowing of the blood vessels by edema or cell swelling that is primarily responsible for "no-reflow". Blood aggregation is a function of the third power of hematocrit and the square of fibrinogen concentration during low flow or no flow.) Nonetheless, cryonics experience indicates that there appears to be reduced clotting seen hours later when heparin has been given at least as much as 15 minutes following ischemia (in the body -- the Fischer experiments were with brains). The half-life of heparin is generally estimated to be 90 minutes, although high doses (400 IU per kilogram) can extend the half-life by as much as an additional hour.
|
Thrombolytic ("clot-busting") agent streptokinase is administered up to 24 hours after a myocardial infarction reduced the chance of death about 22% [EUROPEAN HEART JOURNAL; Yusuf,S; 6:556-585 (1985)]. As little as 250,000 IU of streptokinase could reduce plasma fibrinogen to 30% of the starting level [EUROPEAN HEART JOURNAL; Verstruete,M; 6:586-593 (1985)].
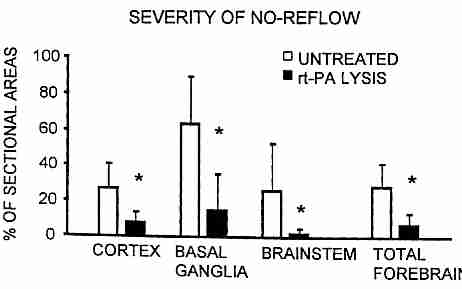
In experiments with cats, combining heparin with thrombolytic recombinant tissue type plasminogen activator (rt−PA) given during CPR after 15 minutes of cardiac arrest resulted in significant reduction of "no−reflow" in the brain: from about 63% to 15% no−reflow in the basal ganglia, 27% to 8% reduction in the cerebral cortex and 26% to 2% reduction in the brainstem [INTENSIVE CARE MEDICINE; Fischer,M; 22:1214-1223 (1996)].
A review of the literature on the use of thrombolytics (mostly rt−PA, recombinant tissue Plasminogen Activator) in CPR, patients who had received thrombolytics had about 40% better survival after 24 hours and at hospital discharge. Patients receiving thrombolytics during hospital treatment for cardiac arrest had over 25% better survival than those who did not [MINERVA ANESTESIOLOGICA; Spohr,F; 71(6):291-276 (2005)]. The use of thrombolytics for stroke victims has been more controversial because of concern about potential promotion of hemorrhage by thrombolytics. Nonetheless, a review of the literature concluded that for rt−PA given within the first 1.5 hours the chance of a 3-month favorable outcome was 2.8 times better. If rt−PA was given between 1.5−3 hours the chance was 1.6 times better, 3−4.5 hours the chance was 1.4 times better and even when given 4.5−6 hours after the stroke the chance was 1.2 times better. Patients receiving rt−PA were nearly 6 times more likely to hemorrhage (irrespective of the time rt−PA was given, more dependent on the age of the patient), but this affected only 6% of the total, so the net risk only begins to match the net benefit beyond 3 hours [LANCET; Hacke,W; 363:768-774 (2004)].
(For more on mechanisms of reperfusion injury, see Reperfusion injury and "No-Reflow". )
In the mid-1970s Dr. Peter Safar was able to prevent brain damage in dogs following 12 minutes of room temperature ischemia by the use of heparin, dextran−40 hemodilution and elevation of blood pressure to 150-180 mmHg with epinephrine for six hours [ARCHIVES OF NEUROLOGY; Safar,P; 33(2):91-95 (1976)]. In the mid-1990s cryonicist Mike Darwin reportedly extended the tolerable room temperature ischemic period to 17 minutes by similar methods and a generous use of anti-oxidants. In both cases the dogs were held at 37ºC (normal human body temperature) rather than 38ºC−38.5ºC (normal dog body temperature), but Darwin's group was reportedly able to achieve their results without the use of elevated blood pressure. Vasopressin may have been of value, however. Unlike epinephrine, vasopressin is active and can elevate blood pressure in acidic conditions (as in many cryonics patients).
Cooling of a cryonics patient with ice immediately following pronouncement of death is the most frequently practiced cryonics rescue procedure. It is commonly noted that metabolic rate is halved for every 10ºC drop in temperature. But the reduction of lipid peroxidation by cooling is greater than the reduction of metabolic rate. Experiments on gerbils indicate that a drop in temperature from 37ºC to 31ºC nearly triples the amount of time that neurons can tolerate ischemia [CRITICAL CARE MEDICINE; Takeda,Y; 31(1):255-260 (2003)]. Human ventricular fibrillation victims subjected to only 1ºC of hypothermia showed a substantial increase in both survival and favorable neurological outcome [CRITICAL CARE MEDICINE; Don,CW; 37(12):3062-3069 (2009)]. Temperature reduction greatly reduces ischemic oxidative stress in mice [FREE RADICAL BIOLOGY & MEDICINE; Khandoga,A; 35(8):901-909 (2003)]. Rats subjected to 2 hours of brain tissue ischemia showed a significant reduction in neurological deficit as a result of only a 4ºC reduction of body temperature for 5 hours, which began one hour following the start of reperfusion [STROKE; Kollmar,R; 33(7):1899-1904 (2002)].
The use of mild hypothermia after cardiac arrest and other types of trauma is increasingly gaining acceptance in conventional medicine [SCIENCE; 317:743-745 (2007)]. In a study of 137 control and 136 hypothermic patients it was found that reduction of body temperature from 37ºC (normal human body temperature) to 32−34ºC for a 24 hour period following cardiac arrest increased 6 month survival from 41% (control) to 55% (hypothermic) and reduced 6 month brain damage from 61% (control) to 45% (hypothermic) [NEW ENGLAND JOURNAL OF MEDICINE; 346(8):549-556 (2002)]. A review of randomized controlled trials using mild hypothermia (32−34ºC) in post cardiac arrest patients showed reduced hospital mortality and improved neurological outcome without any sign of side effects [CANADIAN JOURNAL OF EMERGENCY MEDICINE; Cheung,KW; 8(5):329-337 (2006)].
|
Dog brains stored for up to 4 hours at about 2ºC showed restoration of cerebral electrical activity upon rewarming [NATURE; White,RJ; 209:1320-1322 (1996)]. Experiments with dogs that have been quickly cooled with blood washout solution have shown that cooling from 30ºC to 10ºC can extend the tolerable period of cardiac arrest without neurological damage from 5 minutes to as much as 120 minutes [CRITICAL CARE MEDICINE; Behringer,W; 31(5):1523-1531 (2003)]. Although it is speculative extrapolation, this could mean that a cryonics patient who could tolerate 5 hours of room temperature without much loss of memory or identity prior to a straight-freeze could tolerate about 20 X 5 = 100 hours, or over 4 days, at 10ºC without much loss. But whatever the quantitative effect, it seems certain that cooling would significantly slow the process of apoptosis and it seems likely that any future science that could repair damage caused by disease, aging and toxic cryoprotectants would be expected to reverse the effects of an apoptotic process that has not proceeded too far.
By extrapolation one might expect that tolerable cardiac arrest time could be extended to about 3 hours by cooling to 0ºC (ice water temperature). But between 10ºC and 0ºC the matter could be complicated by the effect of chilling injury, a form of damage probably related to membrane-lipid phase transitions and cold-denaturation of proteins. Profound hypothermia is also associated with capillary damage from erythrocyte clumping. Dextran inhibits red blood cell clumping.
In 1995 dogs were cooled to below 10ºC for over 3 hours and rewarmed with full recovery using new Hyperthermosol® blood washout solutions. Initial blood washout was with "extracellular-type" solution similar to Viaspan® (UW solution), but this was followed by an "intracellular-type" solution (hypertonic solution with low Na+ and high K+) -- which was in turn washed-out by the extracellular-type solution upon rewarming prior to restoration of blood to the animals [CIRCULATION; Taylor,MJ; 91(2):431-444 (1995)]. Intracellular-type solutions have proven to improve cell survival during hypothermic storage [CELL TRANSPLANTATION; Baust,JM; 10(7):561-571 (2001)].
Cold perfluorocarbons have been experimented with as a means of rapidly cooling cryonics patients, either through the lungs or blood vessels. Even at normal body temperature hemodilution with perflurocarbon has been shown to effectively deliver oxygen without impairment of microvasculature [AMERICAN JOURNAL OF PHYSIOLOGY; Cabrales,P; 287(1):H320-H330 (2004)]. When used in the lungs, perfluorocarbons reduced inflammatory response [ANNALS OF THORACIC SURGERY; Jiang,L; 82(1):124-130 (2006)].
It may be unreasonable to expect cooling to greatly extend the period of time over which heparin is effective as an anti-coagulant. Although the biochemical mechanisms that result in a clot (meshwork of fibrin fibers that entrap blood cells, platelets and plasma) can be slowed, cold plasma causes blood cells to aggregate much more readily than warm plasma does. And although the head can be cooled more rapidly than the body, blood from the body will continue to enter the head as long as circulation is maintained.
Cryonics organizations are commonly confronted with cases where potential patients have experienced many hours or days of ischemic damage. The question arises, how much ischemic damage can be tolerated before cryonics is not worth attempting. For warm ischemia (room temperature ischemia), this review would indicate that 9−24 hours has some hope of recovery of memory & identity if freezing damage can be repaired by future technology.
People who deanimate in hospitals are typically placed in refrigeration units if they are not removed immediately by a funeral director. Hospitals tightly manage their space and also realize that there are other reasons not to have dead bodies lying in hospital beds, so the time legal death and movement to a refrigeration unit is usually not very long. Refrigerator temperatures are usually in the range of 2ºC−7ºC (35ºF−45ºF). Based on evidence presented in this essay, the tolerable ischemic time in a hospital refrigerator could be at least 20 times that seen for warm ischemia, meaning 10 days or more. Unfortunately, this estimate is complicated by the fact that the growth of pathogens is inevitable in such a situation. Unpublished studies by cryonicists indicate that 5 days may be the maximum tolerable without bacterial autolysis.
Under less optimistic scenarios -- in which freezing damage is not deemed reparable by future technology -- the critical period will be based on the ability to perfuse vitrification cryoprotectant into the cryonics patient. There is little or no data on reperfusion injury caused by vitrification perfusion, but "no-reflow" would be the greatest concern because of impeded perfusion. A frequent rule-of-thumb in cryonics is that perfusion is too difficult to attempt after 45−60 minutes of warm ischemia (without heparin), although there is not much hard data that can be used to support this rule. At hospital refrigerator temperature the time would probably not be 20 times as great because over this time span more serious consideration should be given to the time for the patient to cool to refrigerator temperature (about 2 hours). If the standard rule is applied, perfusion with vitrification solution would probably be too difficult after about 10 hours in a hospital refrigerator. Again, the growth of pathogens would presumably reduce the tolerable period somewhat.
Evaluating the reparability of ischemic damage by future technology is necessarily guesswork, but with better data (and better analysis of existing data), somewhat reasonable distinctions may be possible between brain tissue that is destroyed (not reparable) and brain tissue that is damaged (and thus potentially reparable in the future). It is always best (most conservative) to minimize current damage/destruction, insofar as we cannot know the capabilities of future science and we cannot always know how well we are preserving reparable tissue. But being conservative also means not deciding the situation is hopeless after a few hours of ischemia, especially when there is evidence to the contrary. There can be no exact cut-off time given between what is hopeless and what is not hopeless, which creates a very difficult problem scientifically, ethically and emotionally.
(For a detailed description of the molecular mechanisms of ischemia & reperfusion injury, see my essay Ischemia and Reperfusion Injury in Cryonics.)