by Ben Best
Mercury is present in the environment as (1) pure metal, (2) inorganic salt and (3) organic mercury.
Elemental metal mercury (Hg0) -- such as was formerly used in thermometers (due to a melting point of -39ºC) -- is not normally toxic when swallowed due to negligible absorption by the gastrointestinal tract. Elemental liquid mercury (quicksilver) is not absorbed in the gastrointestinal tract. Centuries ago a tablespoonful of quicksilver was regarded to be a treatment for constipation. Mercury vapor inhalation can be hazardous, however. It is very readily absorbed by the lungs and migrates quickly into red blood cells and the brain. In the brain Hg0 is oxidized by catalase enzyme to Hg2+ and binds readily to nucleophiles, including glutathione. Hg2+ is nearly a hundred times more toxic than Hg0. Mercury inactivates many enzymes by attaching to sulfur atoms. The "sodium pump" (Na+/K+-ATPase enzyme) is particularly sensitive to mercury. Muscle tremors -- especially of highly innervated muscles (fingers, eyelids, lips) -- progress to trembling & spasms associated with memory loss, excitability and delerium. The shaking and slurred speech was known as "hatter's disease" ("mad as a hatter") when inhalation of mercury vapor was common among those making felt hats.
Inorganic mercury (usually mercuric, Hg2+, salts) most often target the kidney. Mercuric chloride [HgCl2] is toxic to renal tubular lining cells. In the 19th century hat industry rabbit or beaver pelt was dipped into solutions of mercury nitrate [Hg(NO3)2] as a means of getting short hairs of the fur to mat together, contributing to "hatter's disease". Mercurous chloride [calomel, Hg2Cl2] was used to cure syphilis for centuries, but is nearly as toxic to humans as it is to to Treponema pallidum (the organism which causes the disease).
Organic mercury is most commonly found in the environment as methyl mercury [CH3Hg(II)X, MeHg]. Elemental mercury is naturally released into the atmosphere by degassing of the earth's crust, but currently about 70% of the addition of mercury to the atmosphere is due to human industry and the burning of fossil fuels (especially coal-burning plants). Since 1950 there has been increasing artisanal gold mining (panning, etc.) which has increasingly become a major source of mercury pollution worldwide — rising from neglible amounts in 1950 to about 37% in 2010 (versus 26% from fossil fuels, 12% from metal production, 9% from cement production, and 5% from large-scale gold mining [SCIENCE; Lubick,N; 341:1442-1445 (2013)]. Aquatic anaerobic bacteria convert elemental mercury to MeHg, which is taken-up by other microorganisms. Tiny fish eat the microorganisms, other fish eat the small fish, big fish eat the other fish and humans eat the big fish -- with MeHg concentrated more at every step.
Evidence has been presented of reduced cardiovascular disease in people consuming fish high in omega-3 polyunsaturated fatty acids [NEW ENGLAND JOURNAL OF MEDICINE 336:1046 (1997)]. However, another study found just the opposite -- increased cardiovascular disease with increased fish consumption [CIRCULATION 91:645-655 (1995)]. In the latter case, the fish were from freshwater Finnish lakes where the mercury content is particularly high. A study of bovine endothelial cells indicated that MeHg releases inflammatory factors that can induce cardiovascular disease [INTERNATIONAL JOURNAL OF TOXICOLOGY; Mazerik,JN; 26(6):553-569 (2007)].
Methylmercury eaten by humans is almost completely absorbed from the digestive tract and easily accumulates in the brain. MeHg from fish appears to cause neurological damage while increasing the risk of myocardial infarction [SCIENCE 301:1203 (2003)]. The neurotoxic effects seen in adults are considerably greater in the developing fetus. MeHg has a half-life of about 74 days, but the neurodegeneration associated with acute and chronic exposure typically has a latancy period before manifestation [ENVIRONMENTAL HEALTH PERSPECTIVES; Weiss,B; 110(Suppl 5):851-854 (2002)].
MeHg has a high affinity for thiol (-SH, sulfhydryl) groups, making protein & peptides containing the amino acid cysteine vulnerable to structural & functional modification. A particularly high affinity is seen for tubulin sulfhydryl groups in microtubules. Microtubules, which carry vital substances to various parts of a cell, are particularly critical for neuron function and for brain development. (Many substances manufactured in the neuron cell body must be transported down long axons or dendrites through microtubules -- and other substances travel in the other direction.) Necrosis (death) of neurons results in replacement of neurons by glial cells (gliosis).
MeHg can also damage brain tissue by accumulating in astrocytes -- glial cells that normally soak-up glutamate neurotransmitter from synapses. With MeHg accumulation, astrocytes swell -- and astocyte uptake of glutamate is inhibited. Elevated glutamate in the extracellular space may trigger or accelerate neurodegeneration due to excitotoxicity. These effects are more acute for newborns than for adults [TOXICOLOGICAL SCIENCES; Farina,M; 75(1):135-140 (2003)].
Adult MeHg poisoning is characterized by damage to specific brain areas, such as the visual cortex or the granule layer of the cerebellum. Symptoms include visual & hearing abnormalities, muscle weakness, tremor and mental deterioration. Damage in the brain of a developing foetus, by contrast, tends to be more diffuse & widespread. The fetal brain is much more sensitive to low doses of MeHg than the brain of an adult -- and may be damaged even if the mother has no symptoms -- because of interaction of MeHg with DNA & RNA actively involved in synthesis in the developing brain.
Mercury can increase production of superoxide anions. One study showed that mercuric ions (1-6 micromole/Litre) caused up to a 5-fold increase in hydrogen peroxide production in mitochondria -- which is the substrate for the hydroxyl-radical producing Fenton reaction. Mercury's high affinity for sulfhydryl groups inactivates glutathione (which normally regenerates tocopherol from tocopheroxyl radical). Mercury also forms insoluble mercury selenide, thus removing the selenium which could normally act as a co-factor for scavenging of hydrogen peroxide and lipid peroxides by glutathione peroxidase. The iron chelator (iron-binder) deferoxamine can significantly reduce oxidative damage.
Hair level of MeHg is very reliable indicator brain level of MeHg. Hair is a far more sensitive and reliable indicator of MeHg exposure than it is to exposure to other metals -- or even to metallic mercury. A positive correlation was seen with fish intake, hair mercury and urinary mercury. Men who consumed 30 grams of fish per day or more had 56% higher mean mercury hair content and a 2.4-fold risk of coronary mortality compared to men consuming less fish. Women consuming fish show MeHg hair levels 3 times that of non-consumers [ENVIRONMENTAL HEALTH PERSPECTIVES; McDowell,MA; 112(11):1165-1171 (2004)]. A weak association was also seen between cigarette smoking and hair mercury, attributed to intake of mercury from cigarette smoke. Childhood IQ has been negatively correlated with the amount of mercury in the hair of children's mothers [ENVIRONMENTAL HEALTH PERSPECTIVES; Axelrad,DA; 115(4):609-615 (2007)]. Finnish men with high hair mercury content have significantly elevated cardiovascular disease risk [ARTERIOSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY; Virtanen,JK; 25(1):228-233 (2005)].
According to SCIENCE [278:1904-1905 (1997)] a 60 kg woman consuming 4 ounces of fish per week containing average mercury content of 0.25 parts per million would receive an exposure of 0.1 microgram per kilogram of mercury per day -- equivalent to the 1996 US Environmental Protection Agency limit of the daily dose that can be safely consumed over a lifetime. Fetal brain damage risk is associated with maternal hair concentration in excess of 10 parts per million (ppm). The concern for women is especially great because during the third trimester of pregnancy, large amounts of omega-6 and omega-3 polyunsaturated fatty acids are mobilized for development of the brain and vascular system [up to 50% of the total fatty acids in the phospholipids of the cerebral cortex and retina consist of docosanhexaenoic acid (DHA) -- an omega-3 fatty acid]. Many have recommended arachidonic acid and DHA supplementation during pregnancy.(For details about the health benefits of omega-3 fats, see my essay Fats You Need.)
Except for cases of extremely high methylmercury exposure, detrimental effects of fish consumption on neurological function have not been demonstrated. The beneficial effects of omega−3 fatty acids may counter the harmful effects of methylmercury from fish [JOURNAL OF THE AMERICAN MEDICAL ASSOCIATION; Mozaffarian,D; 296(15):1885-1899 (2006)].
Fish with an average tissue concentration in the range of 0.3 to 1.5 parts per million of mercury include swordfish, dolphin, mackerel, snapper and tuna. These are primarily ocean fish, rather than freshwater fish. Average mercury levels in freshwater fish in the United States is about 0.13 ppm. In the NorthEastern United States levels typically exceed 0.5 ppm and in up to 25% of cases exceed 1 ppm [BRAIN RESEARCH BULLETIN 55(2) 197-203 (2001)]. According to the EPA (Environmental Protection Agency), the biggest source of mercury water-pollution comes from coal-fired power plants, with waste incinerators being the second largest source. This information could be of use to fish-eaters who are aware of the sources of the fish they are eating and the location of polluters.
Large long-lived predatory fish are higher in the food-chain and have more time to accumulate methylmercury — and are therefore the most toxic. These include shark, swordfish, king mackeral and tilefish. Far safer are shrimp, salmon, pollock and shellfish [ENVIRONMENTAL HEALTH PERSPECTIVES; Ginsberg,GL; 117(2):267-275 (2009)]. In March 2004 a joint FDA/EPA advisory warned against large fish for women immediately prior to, during and immediately following pregnancy. Tuna steak and albacore tuna were reported to have larger quantities of methylmercury than canned light tuna, which the advisory said is safe. The January 2011 issue of CONSUMER REPORTS (page 20) advised pregnant women to avoid all canned tuna entirely, and stated that although most cans of light tuna have only about a third as much mercury as white tuna (albacore), some light tunas have as much as twice as much mercury as albacore. Although tuna concentrate mercury in their bodies, they protect themselves somewhat by also concentrating Selenium.
Because of the vulnerability of the fetal brain to inhibition of division and migration of neurons by mercury, the US Environmental Protection Agency reduced allowable intake of methyl mercury from 0.5 micrograms to 0.1 micrograms per kilogram per day -- approximately to the level of one 200 gram can of tuna per week (EPA-823-R-01-001). The US FDA has recommended that pregnant women, nursing women and young children completely avoid shark, swordfish, tilefish and king mackeral. Whale meat should also be avoided. The Massechusetts Medical Society has stated that coal-fired and oil-fired power plants are a major source of mercury in fish [NEW ENGLAND JOURNAL OF MEDICINE; Patterson,B; 350:945 (2004)].
Early in 2008 the conservation group Oceana released a survey of samples of fish and sushi bought at groceries and restaurants in 26 American cities. Mercury concentration in tuna steaks from groceries averaged 0.68 parts per million, nearly double the FDA's estimate. In tuna sushi mercury concentration was even higher, averaging 0.86 parts per million.
There is commonly a bias by so-called health-authorities against the use of supplements. However a study comparing dietary fish to fish oil supplements [ARCHIVES OF PATHOLOGY AND LABORATORY MEDICINE 129:74-77 (2005)] concluded that supplements are safer than fish. In contrast to the fish, none of the tested supplements contained PCBs (PolyChlorinated Biphenyls) or organochloride pesticides -- and all contained only negligible amounts of mercury. (For more information about PCBs and other freshwater contaminants, see my essay Is TapWater a Health Hazard?).
Tuna used to be one of my favorite foods, but I rarely eat fish these days because of my concern about mercury. I prefer to get my omega-3 fatty acids from linseed (flaxseed), but I have relied on salmon oil capsules for DHA. (For more detail about DHA -- see my essay DHA for Hearts and Minds).
Caribbean voodoo, Cuban Santeria and some European pagan religions make ritualistic use of mercury, which is a potential health hazard [ENVIRONMENTAL HEALTH PERSPECTIVES; Riley,DM; 109(8);779-784 (2001)].
Concerns have been raised that mercury from dental amalgams may be more dangerous than mercury from fish. Dental amalgams are about half mercury, mixed with an alloy powder that is 60% silver, 27% tin and copper. Sodium and chlorine are both highly toxic elements, but the combination is harmless table salt. By analogy the mercury amalgam used for dental fillings needn't be dangerous like elemental mercury. Dental amalgams do release mercury vapor, but methylmercury is much more dangerous. The amount of mercury vapor released by 12 amalgam fillings is more than ten times less than what would be required for a safe work environment, although nicotine-containing chewing gum causes a five-fold increase [ENVIRONMENTAL RESEARCH; Bergdahl,IA; 77(1):20-24 (1998)]. Chewing in general (including any chewing gum) increases mercury vapor release.
The arguments for and against mercury amalgams have not abated, however. From the point of view of total mercury ingested, an estimated 5−10 times comes from amalgams as comes from fish, and autopsy studies have revealed higher mercury concentrations in the brains & kidneys of those who have had amalgam tooth restorations [THE FASEB JOURNAL; Lorscheider,FL; 9(7):504-508 (1995)]. Some adverse effects on mood, cognition and motor function have been associated with high mercury vapor levels in dental professionals [THE FASEB JOURNAL; Echeverria,D; 12(11):971-980 (1998)]. Zinc, rather than mercury may be the cause of neurotoxicity from dental amalgams [JOURNAL OF DENTAL RESEARCH; Lobner,D; 82(3):243-246 (2003)]. Composite dental filling materials of micron-size glass particles bound in resin are a durable and economical alternative, which does not have the toxic risk associated with mercury amalgam.
"Overnight cures" claimed by people who have had mercury amalgams removed are likely a placebo effect in light of the fact that the removal process considerably elevates mercury exposure -- which declines exponentially over a period of two weeks. Transient elevations of blood mercury levels associated with amalgam removal may be a greater health risk than the long term low level exposure associated with leaving the amalgams in place [THE JOURNAL OF TRACE ELEMENTS IN EXPERIMENTAL MEDICINE; Clarkson,TW; 16:321-343 (2003)].
Some people (under 1% of the population) have allergic reactions to mercury amalgams [JOURNAL OF THE AMERICAN DENTAL ASSOCIATION 122(August):54-61 (1991) & 129(April):494-503 (1998)]. Other studies affirm the safety of dental amalgams [TOXICOLOGICAL REVIEWS; Brownawell,AM; 24(1):1-10 (2005) and JOURNAL OF THE AMERICAN MEDICAL ASSOCIATION; Bellinger,DC; 295(15):1775-1783 (2006)].

|
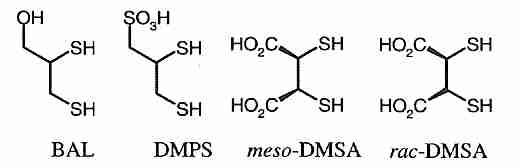
Long-term mercury accumulation in the brain & body can be a concern for anyone, but most particularly for life-extensionists. Chelation therapy may be a way to address this problem, but if done improperly it can be dangerous. (And chelation therapy is only effective against metal ions, not methyl mercury.) The mercury chelator British Anti-Lewisite (BAL = dimercaprol = 2,3−dimercaptopropanol) is more toxic and less soluble than the BAL analogs DMPS (DiMercaptoPropaneSulfonic acid) and DMSA (DiMercaptoSuccinic Acid). DMPS is more is more effective than DMSA or EDTA for removing Hg2+ from renal slices [TOXICOLOGY; Keith,L; 116(1-3):67-75 (1997)], but DMSA and DMPS cannot remove mercury from the brain.
Bacterial enzyme merA can convert the highly toxic Hg2+ to the much less toxic Hg0. And the bacterial enzyme merB can degrade methyl mercury to Hg2+. Transgenic plants that have been given genes for merA and merB can be used for bioremediation of soils contaminated with methyl mercury [CURRENT OPINION IN PLANT BIOLOGY; Meagher,RB; 3(2):153-162 (2000)]. Pretreatment of PC12 cells with nanomolar concentrations of PyrroloQuinoline Quinone (PQQ) protects against methyl mercury toxicity (presumably by anti-oxidant action), but micromolar concentrations of PQQ worsens the toxicity (presumably by pro-oxidant action) [FREE RADICAL RESEARCH; Zhang,P; 43(3):224-233 (2009)].
Selenium deficiency
has been shown to enhance methyl mercury (MeHg) toxicity.
Co-administration of selenium salt with methyl mercury may alleviate mercury
toxicity [THE TOHOKU JOURNAL OF EXPERIMENTAL MEDICINE; Watanabe,C; 196:71-77 (2002)].
MeHg toxicity is primarily related to its binding to thiol (−SH) and
selenol (−SeH) groups on proteins (especially
enzymes) [LIFE SCIENCES; Farina,M; 89(15-16):555-563 (2011)]. MeHg
binds more avidly to the selenol group, and therefore selenium enriched
diets can rapidly reverse severe symptoms of MeHg
toxicity [TOXICOLOGY; Ralston,NVC; 278(1):112-123 (2010)].
(Selenium also enhances antioxidant protection.)
(See also DiMethylMercury and Mercury Poisoning.)