Preparing a cryonics patient for cryostorage can involve three distinct stages of alteration of body fluids:
(1) patient cooldown/cardiopulmonary support
(2) blood washout/replacement for patient transport
(3) cryoprotectant perfusion
During patient cooldown/cardiopulmonary support, a cryonics emergency response team or health care personnel may inject a number of medicaments to minimize ischemic injury and facilitate cryopreservation. The first and most important of these medicaments would be heparin, to prevent blood clotting. (For more details on the initial cooldown process, see Emergency Preparedness for a Local Cryonics Group).
Once the patient is cooled, the blood can be washed-out and replaced with a solution intended to keep organs/tissues alive while the patient is being transported to a cryonics facility. At the cryonics facility the organ/tissue preservation solution is replaced with the cryopreservation solution intended to prevent ice formation when the patient is further cooled to temperatures of −120ºC (glass transition temperature) or −196ºC (liquid nitrogen temperature) for long-term storage.
For both organ/tissue preservation & cryoprotection it is necessary to replace the fluid contents of blood vessels & tissue cells with other fluids. The process of injecting & circulating fluids through blood vessels is called perfusion. The passive process by which fluids enter & exit both blood vessels & cells is called diffusion.

|
Body fluids can be described as solutes dissolved in a solvent, where
the solvent is water and the solutes are substances like sodium chloride (NaCl, table salt),
glucose or protein. Both water and solute molecules tend to move randomly in fluid with
energy and velocity that is directly proportional to temperature. When there is a
difference in concentration between water or solute molecules in one area of the fluid compartment
compared to the rest of the compartment, random motion of the molecules will eventually
result in a uniform distribution of all types of molecules throughout the compartment.
In thermodynamics this is termed a decrease in potential energy (Gibbs free energy, not
heat energy) due to an increase in entropy at constant temperature — leading to equilibrium.
The movement of molecules from an area of high concentration to an area of low concentration is called diffusion. The rate of diffusion (J) can be quantified by Fick's law of diffusion:
dc
J = − DA ----
dx
J = rate of diffusion (moles/time)
D = Diffusion coefficient
A = Area across which diffusion occurs
dc/dx = concentration gradient (instantaneous concentration difference divided by instantaneous distance)
Fick's First Law states that the rate of diffusion down a concentration gradient is proportional to the instantaneous magnitude of the concentration gradient (which changes as diffusion proceeds). For movement of molecules from a region of higher concentration to a region of lower concentration dc/dx will be negative, so multiplying by −DA gives a positive value to J. Diffusion coefficient is higher for higher temperature and for smaller molecules.
Diffusion can occur not only within a fluid compartment, but across partitions that separate fluid compartments. The relevant partitions for animals are cell membranes and capillary walls. Cell membranes are lipid bilayers that allow for free diffusion of lipid soluble substances like oxygen, nitrogen, carbon dioxide and alcohol, while blocking movement of ions and polar molecules. But cell membranes also contain channels made of protein. Protein channels for water allow for very rapid diffusion of water across the membranes. Protein channels for potassium (K+), sodium (Na+) and other ions allow for more restricted diffusion across cell membranes. There is also facilitated diffusion (active transport) of many types of molecules across membranes.
For a normal 70 kilogram (154 pound) adult the total body fluid is about 60% of the body weight. Almost all of this fluid can be described as extracellular or intracellular (excluding only cerebrospinal fluid, synovial fluid and a few other small fluid compartments). Extracellular fluid can be further subdivided into plasma (noncellular part of blood) and interstitial fluid (fluid between cells that is not in blood vessels). Cell membranes separate intracellular fluid from extracellular fluid, whereas capillary walls separate plasma from interstitial fluid. The relative percentages of these fluids can be summarized as:
Intracellular fluid 67%
Extracellular fluid
Interstitial fluid 26%
Plasma
7%
Note that blood volume includes both plasma & blood cells such that adding the intracellular fluid volume of blood cells to plasma volume makes blood 12% of total body fluid.
|
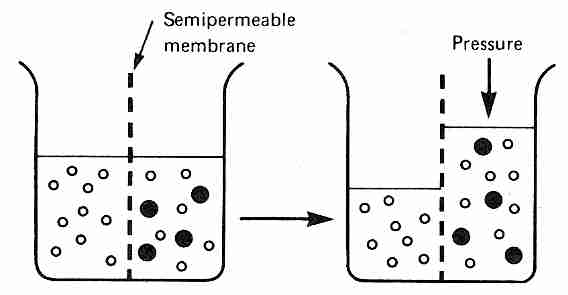
Osmosis refers to diffusion of water (solvent) across a membrane that is semi-permeable, ie, permeable to water, but not to all solutes in the solution. If membrane-impermeable solutes are added to one side of the membrane, but not to the other side, water will be less concentrated on the solute side of the membrane. This concentration gradient will cause water to diffuse across the semi-permeable membrane into the side with the solutes unless pressure is applied to prevent the diffusion of water. The amount of pressure required to prevent any diffusion of water across the semi-permeable membrane is called the osmotic pressure of the solution with respect to the membrane.
Osmotic pressure (like vapor pressure lowering and freezing-point depression) is a colligative property, meaning that the number of particles in solution is more important than the type of particles. One molecule of albumin (molecular weight 70,000) contributes as much to osmotic pressure as one molecule of glucose or one sodium ion. At equilibrium all molecules in a solution have achieved the same average kinetic energy, meaning that molecules with a smaller mass have higher average velocity. Thus, a one molar solution of NaCl will result in twice the osmotic pressure as a one molar solution solution of glucose — because Na+ and Cl− ions exert osmotic pressure as independent particles.
Solute concentrations are generally expressed in terms of molarity (moles of solute per liter of solution). The osmolarity of a solution is the product of the molarity of the solute and the number of dissolved particles produced by the solute. A one molar (1.0 M, one mole per liter) solution of CaCl2 is a three osmolar (three osmoles per liter) solution because of the Ca2+ ion plus the two Cl− ions produced when CaCl2 is added to water. Osmolarity, the number of solute particles per liter has been mostly replaced in practice by osmolality, the number of solute particles per kilogram. (For dilute solutions the values of the two are very close.) For describing solute concentrations in body fluids it is more convenient to use thousandths of osmoles, milli-osmoles (mOsm). Total solute osmolality of intracellular fluid, interstitial fluid or plasma is roughly 300 mOsm/kgH2O. About half of the osmolality of intracellular fluid is due to potassium ions and associated anions, whereas about 80% of the osmolality of interstitial fluid and plasma is due to sodium and chloride ions.
As stated above, both osmotic pressure and freezing point depresssion are colligative properties. All colligative properties are convertible. One osmole of any solute will lower the freezing point of water by 1.858ºC. For this reason, a 0.9% NaCl solution is 0.154 molar or about 308 mOsm/kgH2O, and will lower the freezing point of water by about 0.572ºC.
The osmolality of a solution is an absolute quantity that can be calculated or measured. The tonicity of a solution is a relative concept that is associated with osmotic pressure and the ability of solutes to cross a semi-permeable membrane. Thus, tonicitiy of a solution is relative to the particular solutes and relative to a particular membrane — specifically relative to whether the solutes do or do not cross the membrane. Cell membranes are the membranes of greatest biological significance. Whether a cell shrinks or swells in a solution is determined by the tonicity of the solution, not necessarily the osmolality. Only when all the solutes do not cross the semi-permeable membrane does osmolality provide a quantitative measure of tonicity. It is common to speak as if tonicity and osmolality are equivalent because body fluid solutes are often impermeable. Each mOsm/kgH2O of fluid contributes about 19 mmHg to the osmotic pressure.
A solution is said to be isotonic if cells neither shrink nor swell in that solution. Both 0.9% NaCl (physiological saline) and 5% glucose (in the absence of insulin) are isotonic solutions (roughly 300 mOsm/kgH2O of impermeable solute). (In the presence of insulin, 5% glucose is a hypotonic solution because insulin causes glucose to cross cell membranes.) Hypertonic solutions cause cells to shrink as water rushes out of cells into the solute, whereas cells placed in hypotonic solutions cause the cells to swell as water from the solution rushes into the cells.
An exact calculation of the osmolality of plasma gives 308 mOsm/kgH2O, but the freezing point depression of plasma (−0.54ºC) indicates an osmolality of 286 mOsm/kgH2O. Interaction of ions reduces the effective osmolality. Sodium ions (Na+) and accompanying anions (mostly Cl− & HCO3−) account for all but about 20 mOsm/kgH2O of plasma osmolality. Plasma sodium concentration is normally controlled by plasma water content (thirst, etc.) [BMJ; Reynolds,RM; 332:702-705 (2006)]. Normal serum Na+ concentration is in the 135 to 145 millimole per liter range, with 135 mmol/L being the threshold for hyponatremia. Intracellular sodium concentration is typically about 20 mmol/L — about one-seventh the extracellular concentration. Glucose and urea account for about 5 mOsm/kgH2O. Osmolality of plasma is generally approximated by doubling the sodium ions (to include all associated anions), adding this to glucose & urea molecules, and ignoring all other molecules as being negligible. Protein contributes to less than 1% of the osmolality of plasma. (Cells contain about four times the concentration of proteins as plasma contains.)
Although ethanol increases the osmolality of a solution, it does not increase the tonicity because (like water) ethanol crosses cell membranes. A clinical hyperosmolar state without hypertonicity can occur with an increase in extracellular ethanol (which diffuses into cells) [ MINERVA ANESTESIOLOGICA; Offenstadt,G; 72(6):353-356 (2006)]. Glycerol also readily crosses cell membranes, but it does so thousands of times more slowly than water — which means that glycerol is "transiently hypertonic" (only isotonic at equilibrium). Ethylene glycol crosses red blood cell membranes about six times faster than glycerol (and sperm cell membranes four times faster than glycerol). Actually. even for water there is a finite time for hydraulic conductivity across cell membranes.
|
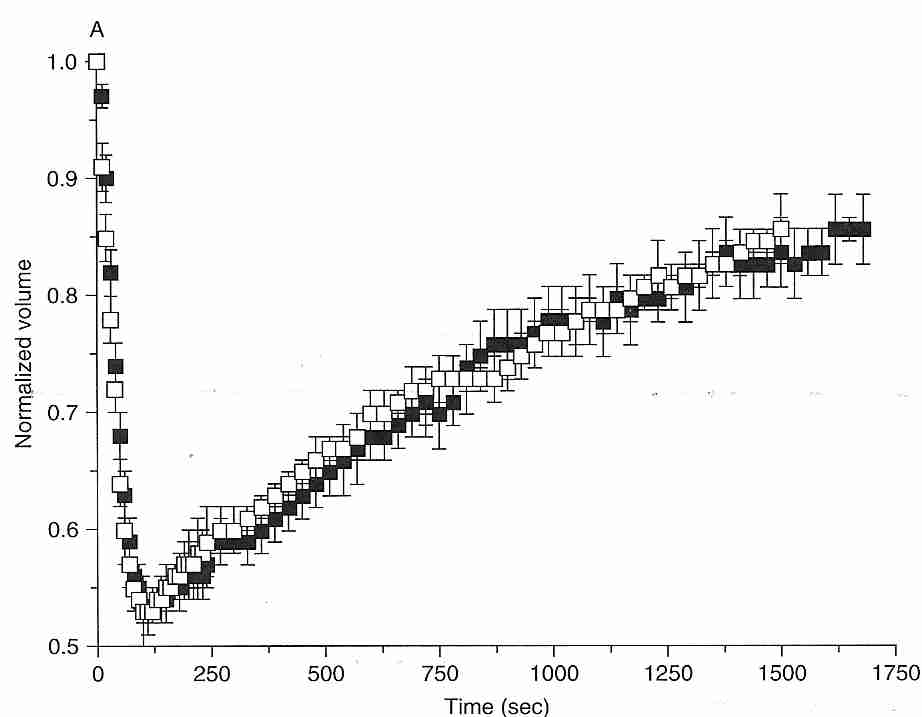
Cells placed in a "transiently hypertonic" solution (containing solutes that slowly cross a membrane) will initially shrink rapidly as water leaves the cell, and gradually re-swell as the solute slowly enters the cell (the "shrink/swell cycle"). As shown in the diagram for mouse oocytes at 10ºC, water leaves the cell in the first 100 seconds, whereas 1.5 Molar ethylene glycol (black squares) or DMSO (DiMethylSulfOxide, white squares) take 1,750 seconds to restore the volume to 85% of the original cell volume [CRYOBIOLOGY; Paynter,SJ; 38:169-176 (1999)]. Even if a cell does not burst or collapse due to osmotic imbalance, a sudden change in osmotic balance can injure cells. Nonetheless, cells are somewhat tolerant of hypotonic solutions. Granulocytes are particularly sensitive to osmotic stress, but granulocyte survival is not significantly affected by hypertonic solutions until the osmolality of impermeant solutes approaches twice physiological values (about 600 mOsm/kgH2O) [AMERICAN JOURNAL OF PHYSIOLOGY; Armitage WJ; 247(5 Pt 1):C373-381 (1984)].
PC3 cells show almost no decline of survival upon exposure to 5,000 mOsm/kgH2O NaCl for 60 minutes at 0ºC, and show nearly 85% cell survival on rehydration. Nearly 85% survive 9,000 mOsm/kgH2O NaCl for 60 minutes at 0ºC, but less than 20% survive rehydration. But although at 23ºC most cells survive exposure to 5,000 mOsm/kgH2O NaCl for 60 minutes, only about a third of cells survive rehydration. At 23ºC and 9,000 mOsm/kgH2O NaCl only about half of cells survive 60 minutes and no cells survive rehydration, indicating the protective effect of low temperature against osmotic stress. Water flux at 23ºC was the same for 9,000 mOsm/kgH2O as for 5,000 mOsm/kgH2O, and hypertonic cell survival was not affected by the rate of concentration increase [CRYOBIOLOGY; Zawlodzka,S; 50(1):58-70 (2005)].
Hyperosmotic stress damages not only cell membranes, but damages cytoskeleton, inhibits DNA replication & translation, depolarizes mitochondria, and causes damage to DNA & protein. Heat shock proteins and organic osmolytes (like sorbitol & taurine) are synthesized as protection against hyperosmotic stress. Highly proliferative cells (like PC3) suffer from osmotic stress more than less proliferative cells because the latter can mobilize cellular defenses more readily due to fewer cells undergoing mitosis at the time of osmotic stress [PHYSIOLOGICAL REVIEWS; Burg,MB; 87(4):1441-1474 (2007)].
An important distinction to remember in replacing body fluids is the distinction between two kinds of swelling (edema): cell swelling and tissue swelling. Cell swelling occurs when there is a lower concentration of dissolved membrane-impermeable solutes outside cells than inside cells. To prevent either shrinkage or swelling of a cell there must be an osmotic balance of molecules & ions between the liquids outside the cell & inside the cell. Capillary walls are semipermeable membranes that are permeable to most of the small molecules & ions that will not cross cell membranes, but are impermeant to large molecules referred to as colloid (proteins). The colloid osmotic pressure on capillary walls due to proteins is called oncotic pressure. For normal human plasma oncotic pressure is about 28 mmHg, 9 mmHg of which is due to the Donnan effect — which causes small anions to diffuse more readily than small cations because the small cations are attracted-to (but not bound-to) the anionic proteins. About 60% of total plasma protein is albumin (30 to 50 grams per liter), the rest being globulins. But albumin accounts for 75-80% of total intravascular oncotic pressure. Tissue swelling occurs when fluids leak out of blood vessels into the interstitial space (the space between cells in tissues). Injury to blood vessels can result in tissue swelling, but tissue swelling can also result from water leaking out of vessels when there is nothing (like albumin) to prevent the leakage.
Both forms of edema (cell & tissue swelling) can impede perfusion considerably, and is frequently a problem in cryonics patients who have suffered ischemic or other forms of blood vessel damage. Maintaining osmotic balance of the fluids outside & inside cells is as important as maintaining oncotic balance, ie, balance of fluids inside & outside of blood vessels.
Much of the isotonicity of the intracellular and extracellular fluids is maintained by the sodium pump in cell membranes, which exports 3 sodium ions for every 2 potassium ions imported into cells. Proteins in cells are more osmotically active than interstitial fluid proteins. Because of the Donnan effect the sodium pump is required to prevent cell swelling. When ischemia deprives the sodium pump of energy, cells swell from excessive intracellular sodium (because sodium attracts water more than potassium does) — resulting in edema. Inflammation can also cause cell swelling due to increased membrane permeability to sodium and other ions. Interstitial edema can occur when ischemia or inflammation increases capillary permeability leading to leakage of larger plasma solutes into the interstitial space.
[For further details on the sodium pump see MEMBRANE POTENTIAL, K/Na-RATIOS AND VIABILITY]
Near the hypothalamus of the brain are osmoreceptors (outside the blood-brain barrier) that monitor blood osmolality, which is normally in the range of 280-295 mOsm/kgH2O. A 2% increase in plasma osmotic pressure can provoke thirst. An increase in plasma osmolality can indicate excessive loss of blood volume. To compensate, the posterior pituitary (neurohypophysis) secretes the hormone 8−arginine vasopressin (AVP), which is two hormones in one — hence the two names vasopressin and anti-diuretic hormone. AVP action on the V1 receptors on blood vessels causes vasoconstriction (vasopressin). AVP action on the V2 receptors of the kidney causes water retention (anti-diuretic hormone). Deficiency in AVP secretion can lead to diabetes incipidus, so called because the excessively excreted urine is tasteless (incipid), in contrast to the sweet (glucose-laden) urine of diabetes mellitus. Cortisol opposes AVP action on excretion, leading to dehydration and excessive urination of fluid. Reduced blood flow to the kidney stimulates release of renin, which catalyzes the production of angiotensin. Like AVP, angiotensin causes vasoconstriction and kidney fluid retention.
Rats subjected to experimental focal ischemia have shown reduced edema when treated with an AVP antagonist [STROKE; Shuaib,A; 33(12):3033-3037 (2002)]. Hypertonic saline (7.5%) has been shown to halve plasma AVP levels in experimental rats, whereas mannitol (20%) had no effect [JOURNAL OF APPLIED PHYSIOLOGY; Chang,Y; 100(5):1445-1451 (2006)]. Increases in plasma osmolality due to urea or glycerol have no effect on plasma AVP levels [JOURNAL OF THE AMERICAN SOCIETY OF NEPHROLOGY; Verbalis,JG; 18(12):3056-3059 (2007)]. The effect of hypertonic saline on osmotic edema due to AVP in a cryonics patient would likely be negligible because of negligible hormone release and transport. So some of the advantage of hypertonic saline over mannitol seen in clinical trials would not occur in cryonics cases.
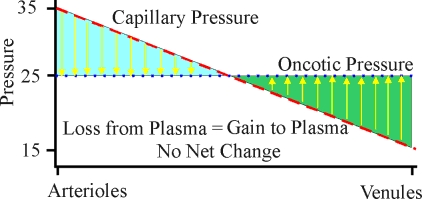
| Variation of Starling forces down a capillary (blood pressure and oncotic pressure) |
|---|
|
The net movement of fluid across capillary membranes due to hydrostatic and oncotic forces can be described by the Starling equation. The Starling equation gives net fluid flow across capillary walls as a result of the excess of capillary hydrostatic pressure over interstitial fluid hydrostatic pressure, and capillary oncotic pressure over interstitial fluid oncotic pressure — modified by the water permeability of the capillary. For a normal (animate) person, the hydrostatic pressure (blood pressure) at the arterial end of a capillary is about 35 mmHg. The hydrostatic pressure drops in a linear fashion across the length of the capillary until it is about 15 mmHg at the venule end. The net oncotic pressure within the capillary is about 25 mmHg across the entire length of the capillary. Thus, for the first half of the capillary there is a net loss of fluid into the interstitial space until the hydrostatic pressure has dropped to 25 mmHg. For the second half of the capillary there is a net gain of fluid into the capillary from the interstitial space. The flow of fluid into the interstitial space in the first half of the capillary is associated with the delivery of oxygen & nutrient to the tissues, whereas the flow of fluid from the interstitial space into the second half of the capillary is associated with the removal of carbon dioxide and other waste products.
Actually, there is a tiny (tiny relative to the total diffusion back and forth across the capillary wall) net flow of fluid from the capillaries to the interstitial fluid — which is returned to the blood vessels by the lymphatic system. The lymphatic vessels contain one-way valves and rely on skeletal muscle movement to propel the lymphatic fluid. Infectious blockage of lymph flow can produce edema. A person sitting for long periods (as during a long trip) or standing a long time without moving may experience swollen ankles due to the lack of muscle activity. Swollen ankles is also a frequent symptom of the edema resulting from congestive heart failure. Venous pressure is elevated by the reduced ability of the heart to pull blood from the venous system, whereas vasoconstriction can better compensate to maintain pressure on the arterial side. Reduced albumin production by the liver as a result of cirrhosis or other liver diseases can reduce plasma osmolality such that the reduced oncotic pressure results in edema — typically swollen ankles, pulmonary edema and abdomenal edema (ascites).
The Starling forces are different for the blood-brain barrier (BBB) than they are for other capillaries of the body because of the reduced permeability to water (lower hydraulic conductivity) and the greatly reduced permeability to electrolytes. The osmotic pressure of the plasma and interstitial fluid effectively become the oncotic pressures.
A critical distinction is made in fluid mechanics between laminar flow and turbulent flow in a pipe. For laminar flow elements of a liquid follow straight streamlines, where the velocity of a streamline is highest in the center of the vessel and slowest close to the walls. Turbulent flow is characterized by eddies & chaotic motion which can substantially increase resistance and reduce flow rate. The Reynolds number is an empirically determined dimensionless quantity which is used to predict whether flow will be laminar or turbulent — with 2000 being the approximate lower limit for turbulent flow. Transient localized turbulence can be induced at a Reynolds number as low as 1600, but temporally peristant turbulence forms above 2040 [SCIENCE; Eckhart,B; 333:165 (2011)].
Turbulent flow could potentially be a problem in cryonics if it reduced perfusion rate or increased the amount of pressure required to maintain a perfusion rate. It is doubtful that turbulent flow ever plays a role in cryonics perfusion, however. Even for a subject at body temperature (37ºC) Reynolds numbers in excess of 2000 are only seen in the very largest blood vessels: the aorta and the vena cava.
The formula for Reynolds number is:
ρ v D
Re = ------
µ
ρ = fluid density (rho)
v = fluid velocity
D = vessel diameter
µ = fluid viscosity
The fact that diameter (D) is in the numerator indicates that only high diameter vessels have high Reynolds number. Velocity (v), also in the numerator, is highest in the aorta & arteries. But the use of cryoprotectants and the increase in viscosity (µ) with declining temperature essentially guarantee that turbulent flow will not occur in a cryonics patient.
More serious for cryonics is the
Hagen-Poisseuille Law, which describes the relationship between
flow-rate and driving-pressure:
pressure X (radius)4
Flow Rate = ----------------------
length X viscosity
Typically in cryonics the flow rate will be one or two liters per minute when the pressure is around 80 mmHg. But because flow rate varies inversely with viscosity and varies directly with pressure, pressure must be increased to maintain flow rates when cryoprotectant viscosity increases with lowering temperature. This poses a serious problem because blood vessels become more fragile with lowering temperature. If blood vessels burst the perfusion can fail.
At 20ºC glycerol is about 25% more dense (ρ=rho, in the numerator) than water. But the role of viscosity is far more dramatic, with high viscosity in the denominator reducing Reynolds number considerably. The viscosity of water approximately doubles from 37ºC to 10ºC, but the viscosity of glycerol increases by a factor of ten (roughly 4 Poise to 40 Poise). At 37ºC glycerol is nearly 600 times more viscous than water, but at 10ºC it is about 2,600 times more viscous.
Although turbulence is not a concern in cryonics, the increase in viscosity of cryoprotectant with lowering temperature certainly is. Fortunately, the newer vitrification mixtures are less viscous than glycerol.
The most common strategy in cryonics has been to cool the patient from 37ºC to 10ºC as rapidly as possible and to perfuse with cryoprotectant at 10ºC. Lowering body temperature reduces metabolism considerably, thereby lessening the amount of oxygen & nutrient required to keep tissues alive. Cryoprotectant toxicity drops as temperature declines. But the very dramatic more-than-exponential increase in cryoprotectant viscosity with lowering temperature poses a significant problem for effective perfusion. When open circuit perfusion is used, a higher temperature may be preferable because the opportunity for diffusion time into cells is so limited (about 2 hours — 1 hour for the head, 1 hour for the body) — although ischemic damage is difficult to quantify.
With closed circuit perfusion, the perfusion times are longer — up to 5 hours. If a good carrier solution is used for the cryoprotectant the tissues may receive adequate nutrient. This, along with the oxygen carrying-capacity of water at low temperature, may limit ischemic damage while allowing time for cells to become fully loaded with cryoprotectant. If ischemic damage can be safely prevented in perfusion, the only critical issues for temperature selection are the relative benefits of reduced cryoprotectant toxicity at lower temperatures as against increased chilling injury. The fact that the more-than-exponential increase in viscosity with lowering temperature will increase perfusion time will not be problematic if the risk of ischemia is minimized.
Typically a cryonics patient deanimates at a considerable distance from a cryonics facility and must be transported before cryoprotectants can be perfused. Blood could be washed-out and replaced with an isotonic (ie, osmotically the same as saline) solution, such as Ringer's solution. The patient is then transported to the cryonics facility at water-ice temperature. Freezing must be avoided because ice crystals would damage cells & blood vessels to such an extent as to prevent effective cryoprotectant perfusion. Water-ice temperature will not freeze tissues because tissues are salty (salt lowers the freezing point below 0ºC).
As body temperature approaches 10ºC, metabolic rate has slowed greatly and the oxygen-carrying capacity of blood hemoglobin is no longer required. Cool water, in fact, may carry adequate dissolved oxygen at low temperatures. (Water near freezing temperature can hold nearly three times as much dissolved oxygen as water near boiling temperature. Oxygen is about five times more soluble in water than nitrogen.) The tendency of blood to agglutinate and clog blood vessels becomes a serious problem at low temperature — so the blood should be replaced if this does not cause other problems (such as delay and reperfusion injury.)
Replacing blood with a saline-like solution for patient transport, however, does not do a good job of maintaining tissue viability or preventing edema and would likely cause reperfusion injury. For this reason an organ preservation solution such as Viaspan®, rather than Ringer's solution, has been used for cryopatient transport. Blood is not simply an isotonic solution carrying blood cells. Blood contains albumin, which attracts water and keeps the water from leaving blood vessels and going into tissues (maintains oncotic balance). Tissues which are swollen by water (edematous tissues) resist cryoprotectant perfusion. One of the most important ingredients in Viaspan® preventing edema is HydroxyEthyl Starch (HES), which attracts water in much the way albumin attracts water — acting as an oncotic agent by keeping water in the blood vessels. Viaspan® contains potassium lactobionate to help maintain osmotic balance. Because HES is difficult to obtain and can cause microcirculatory disturbances, PolyEthylene Glycol (PEG) has been used in organ preservation solutions as a replacement for HES with good results [THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS; Faure,J; 302(3):861-870 (2002) and LIVER TRANSPLANTATION; Bessems,M; 11(11):1379-1388 (2005)].
The same benefit might not apply to cryonics patients, however, because of the prevalence of endothelial damage due to ischemia. Larger "holes" in the vasculature can mean that a larger molecular weight molecule is required for oncotic support. HES molecular weight is about 500,000, whereas the molecular weight for PEG used in organ replacement solutions is more like 20,000. Albumin (which has a molecular weight of about 70,000) provides most of the oncotic support in normal physiology. A PEG with molecular weight of 500,000 would be far too viscous and will form a gel. HES has the benefit of being large enough to always provide oncotic support while being much less viscous than PEG of equivalent molecular weight.
Viaspan® (DuPont Merck Pharmaceuticals) contains other ingredients to maintain tissue viability, such as glucose, glutathione, etc. (the full formula can be found on the Viaspan® website). Viaspan® is FDA approved for preservation of liver, kidney & pancreas, but is used off-label for heart & lung transplants. Viaspan® is being challenged in the marketplace for all these applications by the Hypothermosol (Cryomedical Sciences, BioLife Technologies) line of preservation solutions.
Rather than use these expensive commercial products, Alcor and Suspended Animation, Inc. use a preservation solution developed by Jerry Leaf & Mike Darwin called MHP-2. MHP-2 is so-called because it is a Perfusate (P) which contains mannitol (M) as an extracellular osmotic agent and HEPES (H), a buffer to prevent acidosis which is effective at low temperature. MHP-2 also contains ingredients to maintain tissue viability and hydroxyethyl starch as an oncotic agent to prevent edema. Lactobionate permeates cells less than mannitol and can thus maintain osmotic balance for longer periods of time, but mannitol is much less expensive. Mannitol also has an additional effect in the brain. Because of the unique tightness of brain capillary endothelial cell junctions ("blood brain barrier"), little mannitol leaves blood vessels to pass into the brain. This means that mannitol can act like an oncotic agent for the brain. If the blood brain barrier is intact, mannitol will suck water out of the extravascular space. The brain is the only place that mannitol can do this, and that is why a mannitol is effective for inhibiting edema of the brain — but only if there is not extensive ischemic damage to the blood brain barrier. (Mannitol has yet another benefit in that it scavenges hydroxyl radical [CHEM.-BIOL. INTERACTIONS 72:229-255 (1989)]).
(For the formula of MHP-2 see Table II of CryoMsg 4474 or Table VII of CryoMsg 2874 — which also contains the formula for Viaspan in Table V.)
The initial perfusate can also contain other ingredients to assist in reducing damage to the cryonics patient. Anticoagulants can reduce clotting problems, and antibiotics can reduce bacterial damage. Damaging effects of ischemia can be reduced with antioxidants, antiacidifiers, an iron chelator and a calcium channel blocker.
Both Alcor and Suspended Animation, Inc. use an Air Transportable Perfusion (ATP) system of equipment which allows them to do blood washout in locations remote from any cryonics facilty by using equipment that can easily be carried on an airplane. There is a video demonstration of an ATP on YouTube.
[For further details on organ preservation solution see ORGAN TRANSPLANTATION SOLUTION]
|
Cryoprotectants are used in cryonics to reduce freezing damage by prevention of ice formation (see Vitrification in Cryonics ). Cells are much more permeable to water than they are to cryoprotectant. Platelets & granulocytes, for example, are 4,000 times more permeable to water than they are to glycerol [CRYOBIOLOGY; Armitage,WJ; 23(2):116-125 (1986)]. When a cell is exposed to high-strength cryoprotectant, osmosis causes water to rush out of the cells, causing the cells to shrink. Only very gradually does the cryoprotectant cross cell membranes to enter the cell (the "shrink/swell cycle"). For isolated cells, the halftime (time to halve the difference between a given glycerol concentration in a granulocyte and the maximum possible concentration) is 1.3 minutes [EXPERIMENTAL HEMATOLOGY; Dooley,DC; 10(5):423-434 (1982)] — but tissues & organs would require more time because their cells are less accessible. Even after equilibration, however, the concentration of glycerol inside neutrophilic granulocytes never rises above 78% of the concentration outside the cells.
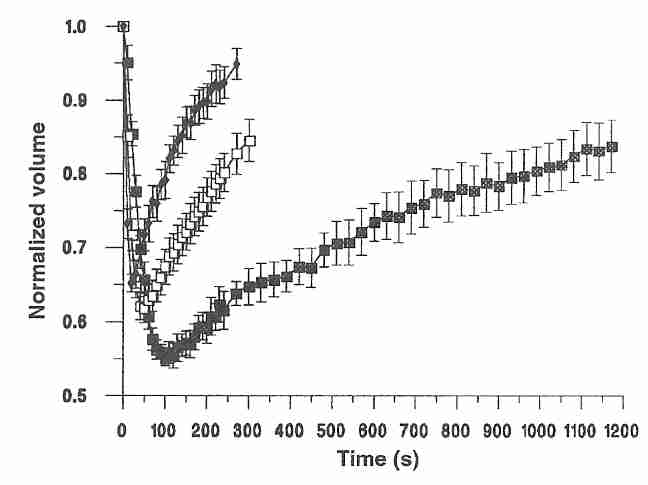
As shown in the diagram for mature human oocytes placed in a 1.5 molar DMSO solution, the shrink/swell cycle is highly temperature dependent, happening with slower speed of recovery and with greater volume change at lower temperatures [HUMAN REPRODUCTION; Paynter,SJ; 14(9):2338-2342 (1999)]. This creates tough choices in cryonics, because cryoprotectants are more toxic at higher temperatures.
Proliferation of cultured kidney cells declines linearly with increasing osmolality due to urea & NaCl above 300 mOsm/kgH2O, but the effect of added glycerol on cell growth is much less [AMERICAN JOURNAL OF PHYSIOLOGY; Michea,L; 278(2):F209-F218 (2000)]. Kidney cells which in vivo can tolerate osmolalities of around 300 mOsm/kgH2O do not survive over 300 mOsm/kgH2O in vitro, possibly because of more rapid proliferation [PHYSIOLOGICAL REVIEWS; Burg,MB; 87(4):1441-1474 (2007)].
Cells subjected to high levels of cryoprotectants can be damaged by osmotic stress. Quantifying osmotic damage has been a challenge for experimentalists who must distinguish between electrolyte damage, cryoprotectant toxicity, cell volume effects and osmotic stress. Concerning the last two, osmotic damage due to cell shrinkage may be distinguished from osmotic damage as a result of the speed at which the cryoprotectant crosses the cell membrane, ie, by the membrane permeability to the cryoprotectant. Cryoprotectants with lower permeabilities can cause more osmotic stress than cryoprotectants with high permeability.
|
Membrane permeabilities of a variety of nonelectrolytes (including
cryoprotectants) have been studied on a number of cell types, including human blood
cells [THE JOURNAL OF GENERAL PHYSIOLOGY; Naccache,P;
62(6):714-736 (1973)]. Critical factors determining membrane permeability
are lipid solubility of the substance (which increases permeability)
and hydrogen bonding (which decreases permeability). In general, permeability
decreases as the molecular size of the substance increases. In contrast
to blood cells, human sperm is more than three times more permeable to
glycerol than to
DMSO [BIOLOGY OF REPRODUCTION; Gilmore,JA; 53(5):985-995
(1995)]. For both blood cells and sperm cells permeability to
ethylene glycol is very high compared to the other common cryoprotectants.
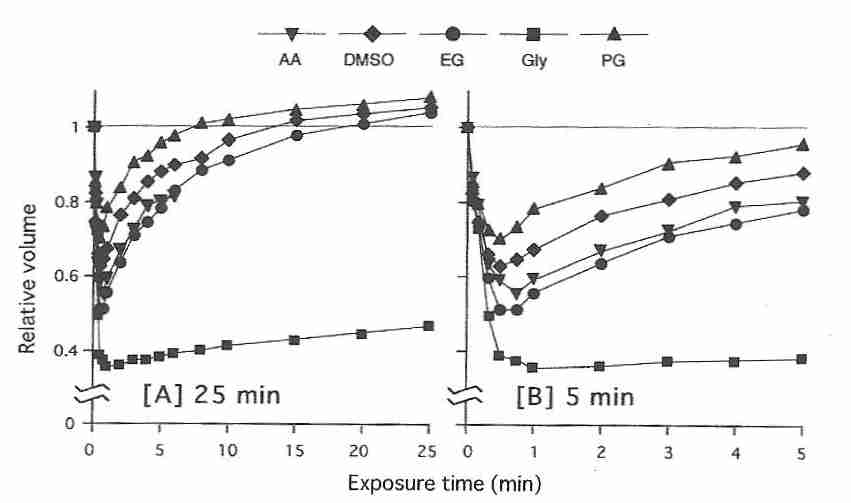
Yet for mature human oocytes propylene glycol has the highest permeability and ethylene
glycol has the lowest permeability of the most commonly used oocyte
cryoprotectants [HUMAN REPRODUCTION; Van den Abbeel,E; 22(7):1959-1972 (2007)].
In contrast to human oocytes, however, for mouse oocytes ethylene glycol (EG)
permeability is comparable to that of DMSO, propylene glycol (PG), and
acetamide (AA), but not
glycerol (Gly) [JOURNAL OF REPRODUCTION AND DEVELOPMENT;
Pedro,PB; 51(2):235-246 (2005)].
|
Water and cryoprotectants both cross cell membranes more slowly at lower temperatures. Cryoprotectants slow the passage of water across cell membranes. Glycerol, DMSO and ethylene glycol all reduce the rate at which water crosses human sperm cell membranes by more than half [BIOLOGY OF REPRODUCTION; Gilmore,JA; 53(5):985-995 (1995)].
Aside from the choice of cryoprotectants, a major concern is
the way cryoprotectant is administered. For example, glycerol (the
standard cryoprotectant used in cryonics for many years) can either
be administered full-strength or it can be introduced
in gradually increasing concentrations. Under optimum conditions,
glycerol results in 80% vitrification and 20% ice formation.
Glycerol has been replaced by better
cryoprotectants that can vitrify without any ice formation, but I will typically
use glycerol as my example cryoprotectant. A patient should probably not be
perfused with a 100% solution of glycerol or other cryoprotectant because
of the possibility of osmotic damage. It is
prudent to begin perfusion with low concentrations of cryoprotectant
because water can diffuse out of cells thousands of times more rapidly than
cryoprotectant diffuses into cells. Using gradually increasing concentrations
of cryoprotectant (ramping) prevents the osmotic damage this differential could
cause.
|
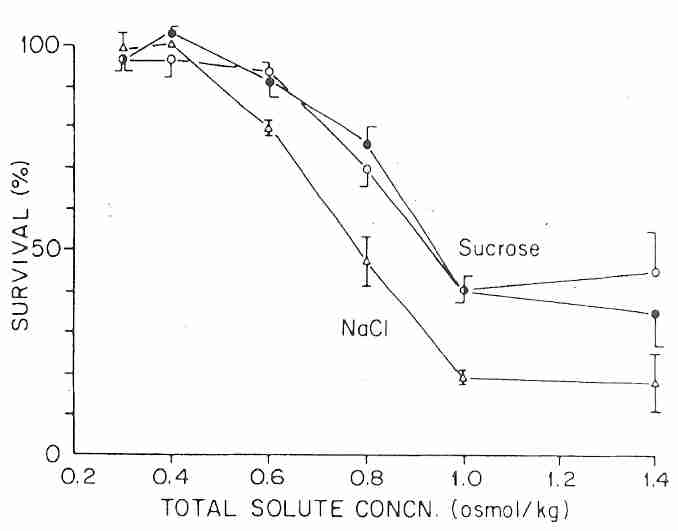
Human granulocytes (which are more vulnerable to osmotic stress or shrinkage than most other cell types) can experience up to 600 mOsm/kgH2O hypertonic solution (which shrinks cells to 68% of normal cell volume) for 5 minutes at 0ºC with no more than 10% of the cells losing membrane integrity. But at about 750 mOsm/kgH2O (NaCl) or 950 mOsm/kgH2O (sucrose) less than half of granulocytes display intact membranes when returned to isotonic solution. Nonetheless, the cells did not display lysis if retained in hyperosmotic medium. In fact, granulocytes could tolerate up to 1400 mOsm/kgH2O if not subsequently diluted to less than 600 mOsm/kgH2O [AMERICAN JOURNAL OF PHYSIOLOGY; Armitage WJ; 247(5 Pt 1):C373-381 (1984)]. A subsequent confirming study showed that rehydration of PC3 cells shrunken by NaCl solution creates more osmotic damage than the initial dehydration [CRYOBIOLOGY; Zawlodzka,S; 50(1):58-70 (2005)]. Cell survival after rehydration was higher at 0ºC than at 23ºC.
Although toxic effects of 2 M (17%w/w) glycerol on granulocytes are quite
evident at 22ºC, almost no toxic effect is seen at 0ºC [CRYOBIOLOGY;
Frim,J; 20(6):657-676 (1983)]. For no mammalian cells other than granulocytes
is 2 Molar glycerol toxic. Nonetheless, abrupt addition of only 0.5 Molar
glycerol at 0ºC resulted in only
40% of granulocytes surviving when slowly diluted to isotonic solution
and warmed to 37ºC. Only 20% of granulocytes survived this treatment
when 1 Molar or 2 Molar glycerol were added (there was no difference
in survival between the two concentrations). But if sucrose or NaCl was
added to keep the granulocytes shrunken to 60% of normal cell volume,
almost all granulocytes survived when incubated to 37ºC. Insofar
as the transient shrinkage of granulocytes due to glycerol is not less than
85% of normal cell volume, it seems unlikely that cell shrinkage
can account for the
damage [AMERICAN JOURNAL OF PHYSIOLOGY; Armitage WJ;
247(5 Pt 1):C382-389 (1984)].
|
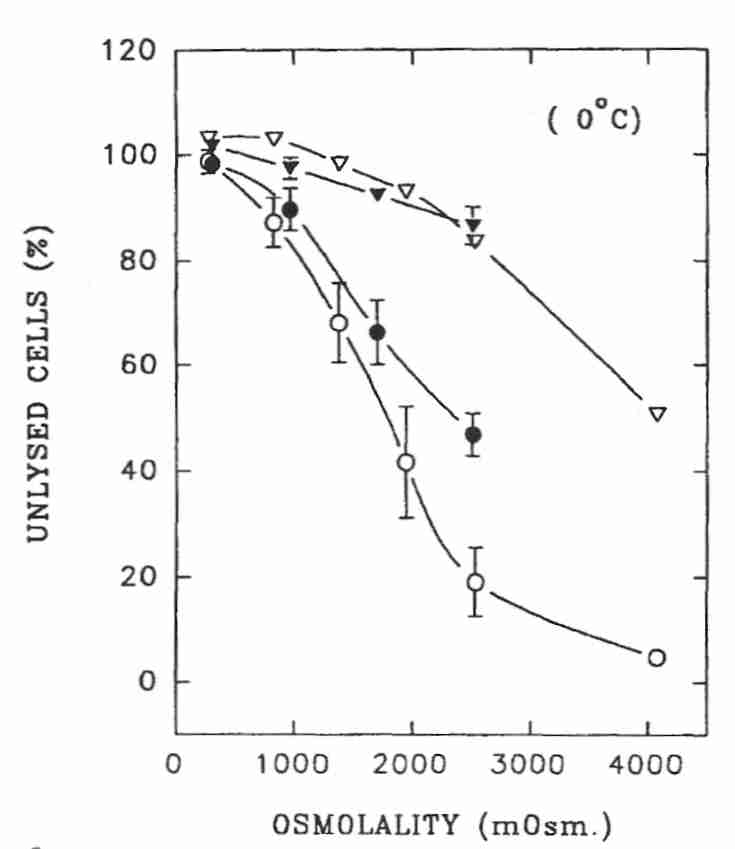
Human spermatazoa tolerate much higher osmolality than granulocytes. Sperm cells can experience up to 1000 mOsm/kgH2O hypertonic solution for 5 minutes at 0ºC with no more than 10% of the cells losing membrane integrity. At about 1500 mOsm/kgH2O (NaCl, white circles) or 2500 mOsm/kgH2O (sucrose, black circles) less than half of sperm cells display intact membranes when returned to isotonic conditions. But 80% of sperm cells showed intact cell membrane after exposure to 2500 mOsm/kgH2O at 0ºC if maintained at hypertonicity rather than restored to isotonic solution (NaCl & sucrose, triangles). Sperm cells gradually returned to isotonic solution following exposure to 1.5 Molar glycerol at 22ºC showed only 3% lysis, whereas 20% of sperm cells lysed if the return to isotonic was sudden. No lysis was seen for sperm not returned to isotonic medium. At nearly 5000 mOsm/kgH2O glycerol (about 4.5 Molar) 17% of sperm cells showed lysis (had loss of membrane integrity) at 0ºC and 10% had lysis at 8ºC if not returned to isotonic media [BIOLOGY OF REPRODUCTION; Gao,DY; 49(1):112-123 (1993)]. For cryonics purposes it would be best to maintain cells in a hypertonic condition to maximize potential viability during cryogenic storage.
Cells from mouse kidney (IMCD, Inner Medullary Collecting Duct) can be killed by NaCl or urea that is 700 mOsm/kgH2O, but the death is apoptotic and takes up to 24 hours. The IMCD cells can tolerate up to 900 mOsm/kgH2O of urea and NaCl in combination because of activation of complementary cellular defenses (including heat-shock protein) [ AMERICAN JOURNAL OF PHYSIOLOGY; Santos,BC; 274(6):F1167-F1173 1998)].
Nearly half of mouse fibroblasts displayed cell membrane lysis after restoration to isotonicity following exposure to the equivalent of 3600 mOsm/kgH2O of osmotic stress from rapid addition of 4 Molar (30%w/w) DMSO at 0ºC. Few cells were damaged by slow addition of the DMSO [BIOPHYSICAL JOURNAL; Muldrew,K; 57(3):525-532 (1990)].
Human corneal epithelial cells could tolerate 4.3 M (37%w/w) glycerol with only 2% cell loss at 4ºC if the cells were subjected to gradually increasing (ramped) concentration (doubling osmolality in about 13 minutes), but for stepped increases of 0.5 M every 5 minutes above 2 M (17%w/w) to 3.5 M (30%w/w) glycerol at 0ºC there was a 27% cell loss. For the same ramped method with DMSO there was a 6% cell loss at 2 M (15%w/w) and a 15% cell loss at 3 M (23%w/w). The same stepped method for DMSO resulted in a 1.5% cell loss for cells stepped from 2 M to 3.5 M (27%w/w) and a 22% cell loss for cells stepped from 2 M to 4.3 M (33%w/w). In all cases cell viability was assessed after washout and three days of incubation at 37ºC [CRYOBIOLOGY; Bourne,WM; 31(1):1-9 (1994)]. (Conversion of glycerol molarity to %w/w was approximated by multiplying by 8.6 and for DMSO was approximated by multiplying by 7.6)
In the context of cryonics it should be remembered that cells are not being returned to body temperature and need not be returned to isotonicity before cryoopreservation. There would be little time for apoptosis, and most cells would be far better preserved at low temperature and in hyperosmolar solution. Future technologies may be able to prevent apoptosis and have better methods for restoring irreplaceable cells to normal temperatures and osmolalities. For neurons, even abrupt stepped perfusion with cryoprotectant is likely to effectively result in ramped perfusion when allowances are made for the diffusion times required across blood vessels (blood brain barrier) and interstitial space. A more worrisome effect from the point of view of cryonic cryoprotectant perfusion is the effect of the cryoprotectants on vessel endothelial cells — notably the effect on edema and vascular compliance.
Cell shrinkage may directly damage the cell (and cell membrane) due to structural resistance from the cell cytoskeleton and high compression of other cell constituents [HUMAN REPRODUCTION; Gao,DY; 10(5):1109-1122 (1995)]. Aside from membrane damage, other forms of cellular damage occur due to hypertonic environments, including cross-linking of intracellular proteins subsequent to cell dehydration. Bull sperm lose motility (often only temporarily) in a less hypertonic medium than one causing membrane damage [JOURNAL OF DAIRY SCIENCE; Liu,Z; 81(7):1868-1873 (1998)]. Osmotic stress can depress mitochondrial membrane potential in a manner that is mostly reversible after restoration to isotonic conditions [PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES (USA); Desai,BN; 99(7):4319-4324 (2002)]. Human oocytes subjected to 600 mOsm/kgH2O sucrose showed 44% of metaphase II spindles having abnormalities [HUMAN REPRODUCTION; Mullen,SF; 19(5):1148-1154 (2004)]. Hypertonic solutions can trigger apoptosis [AMERICAN JOURNAL OF PHYSIOLOGY; Copp,J; 288(2):C403-C415 (2005)].
Despite these other types of damage due to hyperosmolality, the greatest risks in cryoprotectant perfusion in cryonics are those associated with membrane damage and edema due to cell swelling. The evidence that maintaining hypertonicity is more protective of cells than returning to isotonic conditions, and the desire to minimize edema during perfusion seem to make it advisable in cryonics to perfuse in hypertonic conditions.
Once the patient is at the cryonics facility the transport solution can be replaced with a cryoprotectant solution. A perfusion temperature of 10ºC gives the best tradeoff of avoiding the high viscosity of lower temperatures and at the same time limiting the ischemic tissue degradation, chilling injury, and cryoprotectant toxicity that would be seen at higher temperatures. (Cryonicists usually worry more about ischemic damage than cryoprotectant toxicity due to a belief that ischemic damage has a greater likelihood of being irreversible — irreparable by future molecular-repair technology.)
Cryoprotectants should be sterilized to prevent the growth of bacteria. Sterilization of cryoprotectants by heating can cause the formation of carbon-carbon double-bonds, which are evident by a yellowing of the cryoprotectant. Only a few such double-bonds can produce the yellow appearance, so the fact of yellowing is not evidence that the cryoprotectant is no longer serviceable. But a preferable method of cryoprotectant sterilization is filtration through a 0.2 micron filter.
Rapid addition of cryoprotectant causes endothelial cells to shrink — thereby breaking the junctions between the cells [CRYOBIOLOGY; Pollock,GA,; 23(6):500-511 (1986)]. On the other hand, endothelial cell shrinkage by hypertonic perfusate can increase capillary volume, thereby increasing blood flow — as long as excessive vascular damage does not occur. Blood and clots are often observed to be dislodged during cryoprotectant perfusion in cryonics cases. For cryonics purposes some vascular damage may actually be an advantage insofar as it increases diffusion — and vascular repair may be an easy task for future science. In fact, the breakdown of the blood-brain barrier in the 1.8-2.2 molar glycerol range is essential for perfusion of the brain — as long as damaging tissue edema (swelling) can be avoided. Aquaporin (water channel) expression in the blood-brain barrier could be a safer means of allowing cryoprotectants into the brain [CRYOBIOLOGY; Yamaji,Y; 53(2):258-267 (2006)].
Closed-circuit perfusion (with perfusion solution following a circuit both inside & outside the patient's body) is contrasted with the open-circuit perfusion used by funeral directors for embalming. In the open-circuit perfusion of embalming, fluid is pumped into a large artery of the corpse and forces-out blood from a large vein — and this blood is discarded.
A closed-circuit perfusion, as illustrated in the diagram, can be set
up at low cost for gradual introduction of cryoprotectant into cryonics
patients. As shown in the diagram, the perfusion circuit bypasses the
heart. Perfusate enters the patient through a cannula in the femoral (leg) artery
and exits from a cannula in the femoral vein on the same leg. Flowing
upwards (opposite from the usual direction) from the femoral artery and up
through the descending aorta, the perfusate enters the arch of the
aorta (where blood normally exits the heart), but is blocked from entering
the heart. Instead, the perfusate flows (in the usual direction) through
the distribution arteries of the aorta, notably to the head and brain. Returning
in the veins (in the usual direction), the perfusate nontheless again bypasses
the heart and flows downward (opposite from the usual direction)
to the femoral vein where it exits. A better alternative to the femoral circuit,
however, is to surgically open the chest to cannulate the heart aorta (for input)
and atrium (for output).
![[ Closed Circuit Perfusion of a Cryonics Patient ]](./perfuse.gif)
|
Although it is not shown in the diagram, there will be a pump in the circuit to maintain pressure and fluid movement. A roller pump, rather than an embalmer's pump, should be used. A roller pump achieves pumping action by the use of rollers on the exterior of flexible tubing that forces fluids through the tube without contaminating those fluids. Embalmer's pumps may use pressures much higher than those suitable for cryonics, resulting in blood vessel damage. Embalmer's pumps are also easily contaminated (and hard to clean), unless a filter is used. Contamination doesn't matter much in embalming, but in cryonics contaminants entering the patient through the pump can damage blood vessels, interfering with perfusion. If an embalmer's pump is used for cryonics purposes, ensure that the pressure can be lowered to a suitable level and that it is cleaned and sterilized. The main advantage of roller pumps, however, is the fact that they provide a closed circuit, whereas embalmer's pumps are open-circuit. Roller pumps are generally calibrated in litres per minute. Depending on the viscosity of the solution, a flow rate of 0.5 to 1.5 litres per minute will be necessary to achieve the desired perfusion pressure of approximately 80 mmHg to 120 mmHg (physiological pressures).
Gaseous and particulate microemboli can produce ischemia in capillaries and arterioles. A study of patients having routine cardiopulmonary bypass surgery showed that 16% fewer patients had neuropsychological deficits eight weeks after the surgery when a 40 micrometer arterial line filer had been used [STROKE; Pugsley,W; 25(7):1393-1399 (1994)]. Both roller pumps (peristaltic pumps) and centrifugal pumps can generate particles up to 25 micrometers in diameter through spallation, although centrifugal pumps generate fewer particles [PERFUSION; Merkle,F; 18(suppl 1):81-88 (2003)]. Filtration of perfusate with a 0.2 micrometer filter prior to perfusion is a recommended way of removing potential microemboli, including bacteria. At room temperature 20 micrometer diameter air bubbles take 1 to 6 seconds to dissolve in water, although high flow rates and turbulence can increase microbubble formation [SEMINARS IN DIALYSIS; Barak,M; 21(3):232-238 (2008)]. De-airing of tubing before perfusion considerably reduces the possibility of microbubbles entering the patient [THE THORACIC AND CARDIOVASCULAR SURGEON; Stock,UA; 54(1):39-41 (2006)].
Mean Arterial Pressure (MAP) for an normal adult is regarded as being in the range of 50 to 150 mmHg, and Cerebral Perfusion Pressure (CPP) is in the same range [BRITISH JOURNAL OF ANAETHESIA; Steiner,LA; 91(1):26-38 (2006)]. Vascular pressure normally drops to about 40 mmHg in the arterioles, to below 30 mmHg entering the capillaries, and is down to 3 to 6 mmHg (Central Venous Pressure, CVP) when returning to the right atrium of the heart. Perfusing a cryonics patient at about 120 mmHg should open capillaries adequately for good cryoprotectant tissue saturation without damaging fragile blood vessels.
Outside the patient, some of the drainage is discarded, but most is returned to a circulating (stirred) reservoir connected to a concentrated reservoir of cryoprotectant. The circulating reservoir is initially carrier solution which gradually becomes increasingly concentrated with cryoprotectant as the stirring and recirculation proceed. The circulating reservoir can be stirred from the bottom by a magnetic stir bar on a stir table and/or from the top by an eggbeater-type stirring device. The stirring will draw cryoprotectant from the cryoprotectant reservoir, and pumping of the perfusate should also actively draw liquid from the cryoprotectant reservoir. Gradually a higher and higher concentration of cryoprotectant is included in the perfusate and the osmotic shock of full-strength cryoprotectant is avoided.
The carrier solution for the cryoprotectant should perform similar tissue preservation functions as is performed by the transport solution, and should be carefully mixed with the cryoprotectant so as to avoid deviations from isotonicity which could result in dehydration or swelling & bursting of cells. The carrier solution will help keep cells alive during cryoprotectant perfusion.
An excellent carrier solution for cryonics purposes would be RPS-2 (Renal Preservation Solution number 2), which was developed by Dr. Gregory Fahy in 1981 as a result of studies on kidney slices. More recently Dr. Fahy used RPS-2 as the carrier solution in cryopreserving hippocampal slices — an indication that it is well-suited for brain tissue as well as for kidney. RPS-2 not only helps maintain hippocampal slice viability, it reduces the amount of cryoprotectant needed because it has cryoprotectant (colligative) properties of its own. The formulation of RPS-2 is: K2HPO4, 7.2mM; reduced glutathione, 5mM; adenine HCl, 1mM; dextrose, 180mM; KCl, 28.2mM; NaHCO3, 10mM; plus calcium & magnesium [CRYOBIOLOGY; Fahy,GM; 27(5):492-510 (1990)]. LM5 (Lactose-Mannitol 5) is a carrier solution for use in vitrification solutions that include ice blockers. LM5 does not contain dextrose, which is believed to interfere with ice blockers.
The cryoprotectant reservoir will not in general contain pure cryoprotectant (although in principle it could), but rather a "terminal concentration" solution of cryoprotectant that is equal or slightly above the final target concentration. As perfusion proceeds and drainage to discard proceeds, the level of both reservoirs drops in tandem until both reservoirs are nearly empty, at which point the circuit concentration will have reached the cryoprotectant reservoir concentration. Provided that the two reservoirs are the same size and same vertical elevation, the gradient will be linear over time (if the drainage rate to discard was constant).
For cryoprotectant to perfuse into cells there must be constant exposure to cryoprotectant surrounding the cells — and there must be pressure to maintain that exposure. In a living animal the heart maintains blood pressure that forces blood through the capillaries and forces nutrients into cells. A dead animal with no blood pressure — and which is being perfused with cryoprotectant — also requires pressure for the capillaries to remain open and for cryoprotectant to be maintained at high concentrations around cells.
Alcor found that closed-circuit perfusion must be maintained for 5-7 hours for full equilibration of glycerol, because the diffusion rate of water out of cells is thousands of times the rate at which glycerol enters cells. Of course, it would be possible to pump glycerol into a patient for 5-7 hours with open-circuit perfusion, but only by using thousands of dollars worth of glycerol. The newer vitrification cryoprotectants used by Alcor are vastly more expensive than glycerol. When using expensive cryoprotectants it makes far more sense to recirculate in a closed circuit. Closed-circuit perfusion also has the benefit of allowing for ongoing monitoring of physiological changes occurring in the patient's body during the perfusion process. Open-circuit with an inexpensive cryoprotectant has the advantage of avoiding recirculation of toxins.
Cryoprotectants, particularly glycerol, are viscous — and cryoprotectants in high concentration are particularly viscous. The introduction of air bubbles into cryoprotectant solutions during pouring and mixing should be avoided because air emboli that enter the cryonics patient can block perfusion. Elimination of air bubbles from viscous cryoprotectant solutions is extremely difficult. Prevention is more effective than cure. Cryonicist Mike Darwin wrote about this problem and possible solutions in a 1994 CryoNet message.
Improper mixing of perfusate containing high levels of cryoprotectant can result in a phenomenon that appears to be high viscosity, but in reality is edema. If, for example, isotonic carrier solution is mixed half-and-half with cryoprotectant solution an open circuit perfusion may have to be halted when no further perfusate will go into the patient. The problem is caused not by viscosity, but by the fact that the isotonic solution became hypotonic due to dilution with cryoprotectant — causing the cells to swell and forcing perfusion to end. In closed-circuit perfusion, the cryoprotectant concentrate reservoir contains cryoprotectant at about 125% the terminal concentration in a vehicle of isotonic carrier solution so that when reservoir concentrate is mixed with isotonic carrier there is no change in tonicity.
Newer cryoprotectants are less viscous than glycerol, so perfusions can be done in less time. After 15 minutes of perfusion with carrier solution, cryoprotectant concentration linearly increases at a rate of 50 millimolar per minute until full concentration is reached — in about two hours (a protocol developed on the basis of minimizing osmotic damage when perfusing kidneys). Perfusion is increased for an additional hour or two until the cryoprotectant has fully diffused into cells (as indicated by similarity of afflux and efflux cryoprotectant concentrations).
Only after a few hours of closed-circuit perfusion is the concentration of cryoprotectant exiting the cryonics patient equal to the concentration of cryoprotectant entering the patient. Only an extended period of sustained pressure will keep capillaries open, and otherwise facilitate diffusion of cryoprotectant into cells. And the exiting cryoprotectant concentration will equal the entering cryoprotectant concentration only when the tissues are fully loaded with cryoprotectant. A refractometer is used to verify that terminal cryoprotectant concentration has been reached in the brain.
(A refractometer measures the index of refraction of a liquid, ie, the ratio of the speed of light in the liquid and the speed of light in a vacuum (or air). Light changes speed when it strikes the boundary of two media, thus causing a change in angle if it strikes the new medium at an angle. Because the refractive index is a ratio of two quantities having the same units, it is unitless. Sodium vapor in an electric arc produces an excitation between the 3s and 3p orbitals resulting in yellow-orange light of 589nm — what Joseph Fraunhofer called the "D line". Insofar as the sodium "D line" was the first convenient source of monochromatic light, it became the standard for refractometry. The refractive index of a liquid is thus a high-precision 5-digit number between 1.3000 and 1.7000 at a specific temperature, measured at the sodium D line wavelength. For example, the refractive index of glycerol at 25ºC — nD25 — is 1.4730.)
Closed-circuit perfusion may be necessary for removal of water as well as loading of cryoprotectant if it is true that open-circuit perfusion cannot remove water effectively.
One could imagine that the additional time spent doing closed-circuit (rather than open-circuit) perfusion means increased damage due to above-zero temperature. But most cells are still alive and metabolizing very slowly at 10ºC. Viaspan®, RPS-2 and other organ preservation solutions are designed to keep tissues alive for extended periods at near-zero temperatures — certainly for the time required for closed-circuit perfusion. Ramping (slowly increasing concentration) of cryoprotectant should be done in such a way that the ion and mannitol or lactobionate concentration remains unchanged in the perfusate. Ramping is not an osmotically neutral process, however, because cryoprotectant is expected to dehydrate tissues.
Ramping at the rate of 2-4 molar per hour increase of glycerol concentration is slow enough to allow full diffusion of glycerol into tissues. Cell shrinkage due to diffusion of water from cells is followed by slow cell swelling due to the gradual diffusion of glycerol into cells. Dehydration of epithelial cells in the blood vessels by glycerol ramping facilitates perfusion by making the vessels wider. If the ramping rate is too slow, the epithelial cells become loaded with glycerol quickly enough that the vessel-widening advantage is lost.
Care must be taken that the concentration of non-penetrating solutes (such as mannitol) in the carrier solution is not less than what is in the cells — normally 300 mOsm/kgH2O concentration. If the concentration of nonpenetrating solutes in the carrier solution is hypotonic — 150mM, for example — cells will swell to twice their volume irrespective of the glycerol concentration. This swelling would occur first in the epithelial cells, which could seriously impede the process of perfusion. Faster ramping would lessen this effect by dehydration of the epithelial cells, but the increased rate would be too rapid to allow full diffusion of glycerol into the tissues. It is better to avoid these problems by maintaining a carrier solution concentration of nonpenetrating solutes at least 300 mOsm/kgH2O — and probably less than 600 mOsm/kgH2O.
Although glycerol has historically been the cryoprotectant used in cryonics, the perfusion process described should be much the same with the newer, more effective, cryoprotectants now being used in cryonics. Cryoprotectant toxicity varies directly with temperature, but cryoprotectant viscosity varies inversely with temperature. There is therefore a trade-off between perfusing at a higher temperature for more rapid cryoprotectant penetration, and suffering the increased toxicity of higher temperature. Unlike osmotic damage, however, toxicity may affect viability through biochemical rather that structural damage and thereby be of less concern for future repair. Ischemic injury is likely to be minimal due to nutrient & oxygen in the carrier solution. by more time at higher temperature, however, is likely to increase structural damage.
Some cryonics protocols are beginning the introduction of cryoprotectant at 10ºC and finishing at 5ºC. A near-term objective is to be able to introduce half the cryoprotectant at 10ºC and the other half at −10ºC. It seems like a good strategy to add the least toxic components of a cryoprotectant cocktail first (at higher temperatures) and the most toxic components at lower temperature — or simply to increase cryoprotectant concentration as temperature drops. (This technique was used successfully in the Hippocampal Slice Cryopreservation Project.)
| Carotid & Vertebral Artery | Circle of Willis in context | Circle of Willis |
|---|---|---|
|
|
|
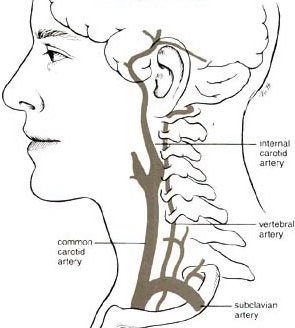
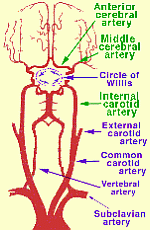
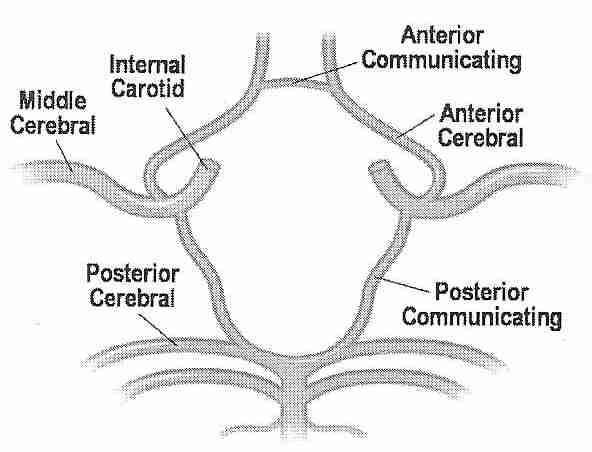
Perfusing heads, rather than whole bodies, can be challenging because of the complex vascular and particular requirements. Two large arteries (the carotid arteries) and two small arteries (the vertebral arteries) supply the head, whereas all of the venous return from the head comes through the jugular veins. The carotid arteries and the vertebral arteries join in the head, however, in the Circle of Willis, which should mean that the vertebral arteries are somewhat redundant, and that full brain perfusion should be possible by simply perfusing into the carotid arteries. But the Circle of Willis is often incomplete. For that reason, a great deal of effort has been made in cryonics to perfuse the head through both the carotid and the vertebral arteries.
One approach has been to cut into the sternum (median sternotomy) to open the chest to gain access to the aorta — so that all head blood vessels can be accessed below where they separate. Clamping the ascending aorta (going to the lower body) and the subclavian arteries (going to the arms) means that perfusing into the ascending aorta will perfuse mainly the head and neck (through the carotids and vertebrals). Clamping the subclavian arteries is not always easy to do, and in some cases tourniquets have been applied to the arms to prevent perfusing them. With this approach, the superior vena cava can be cannulated for effluent sampling of refractive index (to determine brain/head saturation with vitrification solution). Cannulating the superior vena cava is easier than cannulating the jugular veins, but checking refractive index from the jugular veins allows for monitoring vitrification solution saturation of both brain hemispheres independently.
For neuro (head-only) perfusion Alcor currently removes the head from the body ("cephalic isolation") and perfuses the head independently. Perfusion is into the carotids, with drainage from the jugulars collected, filtered and re-circulated back into the carotids. No attempt is made to perfuse into the vertebrals unless perfusate is not seen to be dripping from the vertebrals (which would mean that the Circle of Willis is incomplete — in which case the vertebrals would be cannulated for perfusion.
In one study, Magnetic resonance angiograms of healthy adult volunteers showed a complete Circle of Willis in only 42% of cases — more often complete in younger persons and females [RADIOLOGY; Krabbe-Hartkamp,MJ; 207(1):103-111 (1998)]. In a magnetic resonance angiography study of healthy volunteers in the 65-68 age group, 47% had a complete Circle of Willis [JOURNAL OF CARDIOVASCULAR SURGERY; Macchi,C; 43(6):887-890 (2002)]. A correlation has been seen between magnetic resonance images of incomplete Circle of Willis and deficient blood flow rates in arteries coming from the Circle of Willis [AMERICAN JOURNAL OF NEURORADIOLOGY; Tanaka,H; 27(8):1770-1775 (2006)]. A study which found an incomplete Circle of Willis in 33% of all subjects found that the Circle of Willis was incomplete in 51% of those subjects having migraine headaches [HEADACHE; Bugnicourt,J; 49(6):879-886 (2009)]. In cases of internal carotid artery occlusion, absence of the ipsilateralposterior communicating artery of the Circle of Willis increases the likelihood of ischemic infarction [NEW ENGLAND JOURNAL OF MEDICINE; Schomer,DF; 330(22):1565-1570 (1994)]. Nonetheless, a study that found that the Circle of Willis was complete in 59 of 99 patients found no case of insufficient perfusion in functional tests — concluding that extracranial collateral circulation provides an alternative pathway to the Circle of Willis for cerebral cross-perfusion [EUROPEAN JOURNAL OF CARDIO-THORACIC SURGERY; Urbanski,PP; 33(3):402-408 (2008)]. Good collateral circulation is a favorable prognostic vactor for stroke victims [CURRENT CARDIOLOGY REVIEWS; Romero,JR; 5(4):279-288 (2009)].
For a more detailed analysis of the issues related to the Circle of Willis and patient perfusion, see: The Circle of Willis in Cryonics Perfusion
Insofar as getting cryoprotectants to the brain is a primary
objective of cryonics, understanding the nature of the
"barrier" between the blood plasma and the brain is a matter
of primary importance.
| Typical Capillary Wall | Typical Capillary versus BBB Capillary |
|---|---|
|
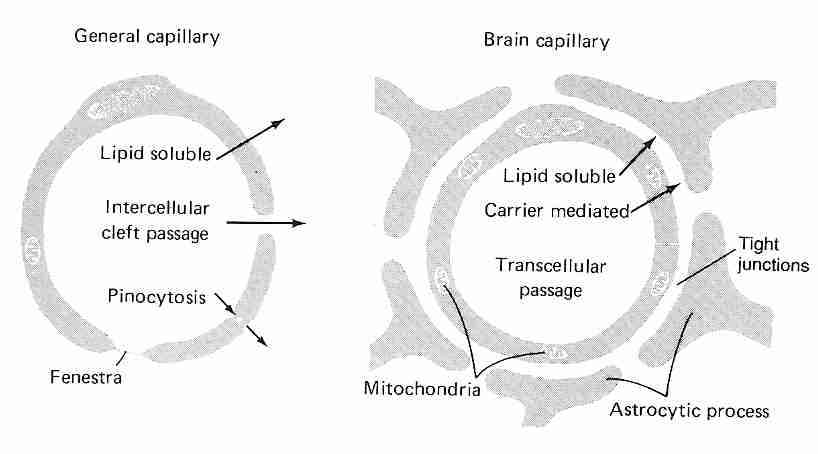
|
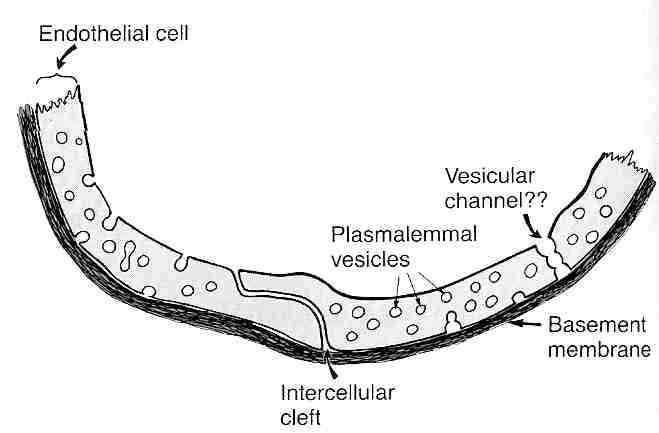
Diffusion through capillary walls is the means by which most substances are transferred between the bloodstream and the interstitial fluid (the fluid between cells in body tissues). Capillaries are thin-walled tubes composed of a single layer of highly permeable endothelial cells lying on a thin basement membrane. Lipid soluble molecules such as ethanol, nicotine & diazepam as well as gases like oxygen & carbon dioxide diffuse directly across the capillary membranes. Gaps between adjacent endothelial cells (intercellular clefts) allow for diffusion of water-soluble substances, as do channels through the endothelial cell walls (fenestrae, "little windows"). Plasmalemma vesicles allow for pinocytosis (vesicle transport) of substances across the membrane. Intercellular clefts in the liver are so wide that plasma proteins can pass directly from the blood into liver tissue. Fenestrae are so large in kidneys that substances can be filtered in the glomeruli without having to pass through intercellular clefts.
| Tight junction, normal versus with glioma |
|---|
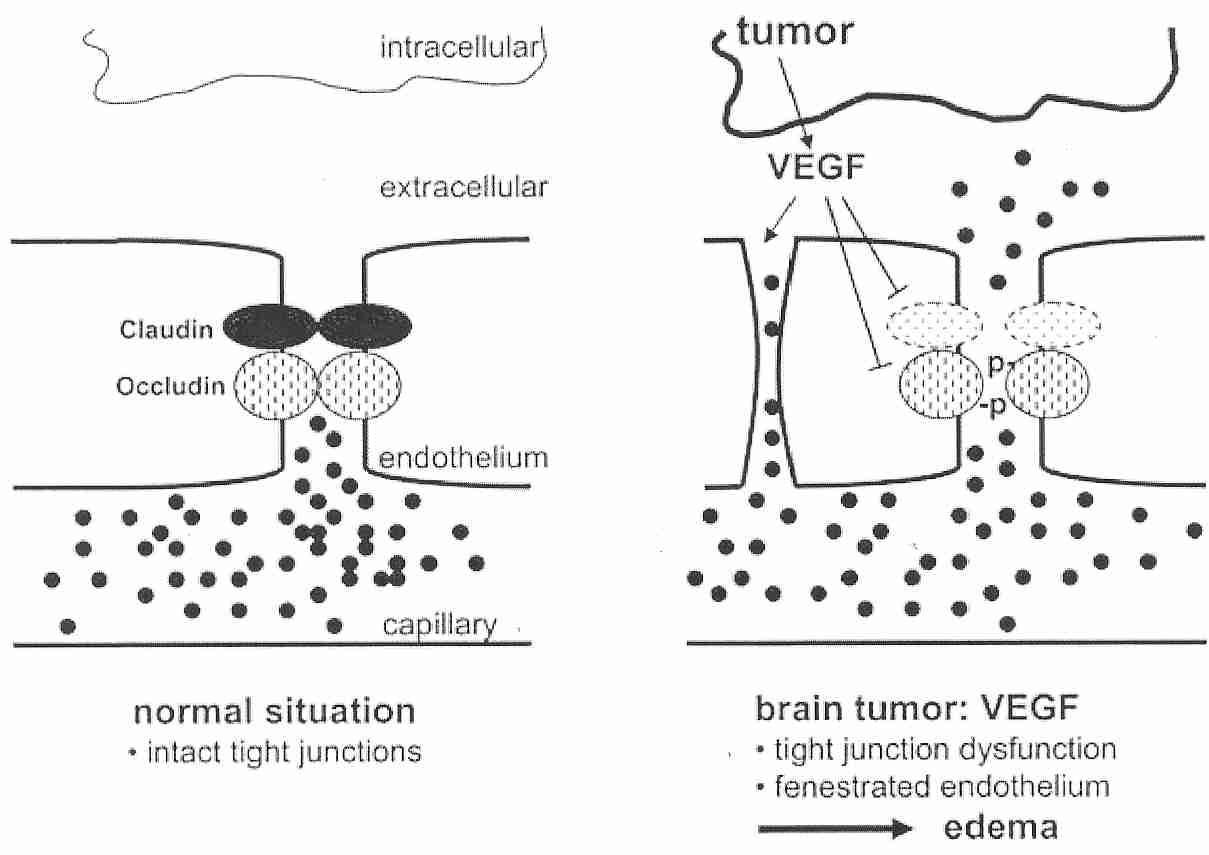
|
By contrast, the endothelial cells in brain capillaries are packed so tightly that there are no intercellular clefts, although tight junctions exist which are regulated by a number of transmembrane proteins that respond to intracellular signalling. The tight junctions have a high electrical resistance, providing a barrier to ions. Moreover, there are no fenestrae and there is little or no pinocytosis in brain capillary endothelial cells [PHARMACOLOGICAL REVIEWS; Hawkins,BT; 57(2):173-185 (2005)]. These tightly packed endothelial cells are the blood-brain barrier (BBB). More specifically, the BBB consists of the luminal endothelial cell membrane facing the bloodstream, the abluminal endothelial cell membrane facing the brain interstitial fluid, and 300 to 500 nanometers of endothelial cytoplasm between these two membranes [NEURORX; Cornford,EM; 2(1):27-43 (2005)].
Only water, small lipophilic molecules and actively transported substances cross the BBB. Because glucose, vitamins and many amino acids can only cross the BBB endothelial cells by active transport, the endothelial cells of the BBB have 2 to 4 times as many mitochondria as other endothelial cells. The mitochondria also assist in maintaining electrochemical gradients and tight junction complexes. The high ATP requirements of the BBB make it particularly susceptible to ischemic injury [AMERICAN JOURNAL OF PHYSIOLOGY; Witt,WA; 285(6):H2830-H2831 (2003)].
The tight junctions connecting cerebrovascular endothelial cells can be called the site of the BBB. The claudin and occludin transmembrane proteins which bind the tight junctions are connected to intracellular cytoskeleton and other proteins. Cancerous glial cells (gliomas) of the brain can have greatly increased secretion of Vascular Endothelial Growth Factor (VEGF) resulting in decreased occludin synthesis and increased vascular permeability (causing edema). Glucocorticoid treatment has been shown to reduce VEGF expression in malignant gliomas [JOURNAL OF CLINICAL INVESTIGATION; Heiss,JD; 98(6):1400-1408 (1996)]. Within six hours of a stroke, hypoxia in the ischemic area border zone induces VEGF expression, resulting in edema and re-vascularization [NATURE; Weis,SM; 437:497-503 (2005)].
The BBB provides the brain with a chemical environment that is somewhat more stable than — and somewhat independent of — the chemical environment provided to the rest of the body by the bloodstream. The BBB protects the brain from variations of ions, amino acids, sugars, hormones, etc in the bloodstream due to diet, hormone release and other factors. Many amino acids such as glycine and GABA (Gamma-AminoButyric Acid) which act as neurotransmitters in the brain are markedly restricted by the BBB to prevent entry into the brain. Facultative transport of glutamine and glutamate occurs in the luminal — but not the abluminal — membrane of the BBB, facilitating removal of thes amino acids from the brain [PHARMACOLOGIAL REPORTS; Bernacki,J; 60(5):600-622 (2008)]. Norepinephrine in the bloodstream secreted by the adrenal medulla could significantly affect brain activity, but this polar molecule only slowly crosses the BBB, and the brain is thus protected from variations in plasma levels of that hormone that also acts as a neurotranmitter.
| Astrocyte with end-feet on capillary and neuron |
|---|
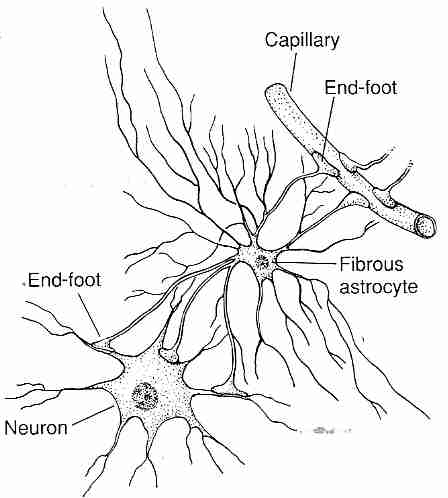
|
Astrocytes are brain glial cells which have "end-foot" processes connected to neurons and BBB endothelial cells, providing biochemical support. The astrocyte processes surround BBB capillaries so completely that they were once thought to be the BBB, although it is now known that they are not. Astrocytes may, however, transmit biochemical signals to the BBB endothelial cells which helps induce the BBB endothelial cell phenotype. Pericytes also affect endothelial cells by inducing a capillary-like structure [THE FASEB JOURNAL; Ramsauer,M; 16(10):1274-1276 (2002)] and regulating blood flow by means of capillary diameter changes [ NATURE; Peppiatt,CM; 443:700-704 (2006)].
The BBB actively transports D−glucose rather than its stereoisomer L−glucose. Water crosses the BBB less readily than it crosses other capillary walls, protecting the brain from changes in plasma tonicity. The BBB is only slightly permeable to electrolytes, such as sodium, potassium and chloride — as impermeable as some cell membranes. Potassium was determined to permeate the BBB at a rate of 13.5 x 10−7 centimeters per second, nearly ten times as fast as sodium (which permeates slightly faster than chloride) [JOURNAL OF NEUROCHEMISTRY; Smith,OR; 46(6):1732-1742 (1986)]. Electrically neutral molecules with a molecular mass less than 400-500 atomic mass units can cross the BBB, but permeation decreases exponentially as molecular size increases for non-lipophilic atoms [MOLECULAR INTERVENTIONS; Pardridge,WM; 3(2):90-105 (2003)]. Substances with more than 8-10 hydrogen bonds show minimal transport across the BBB [NEURORX; Pardridge,WM; 2(1):3-14 (2005)].
Ischemic BBB damage allows for greater passage of cryoprotectant, although hypothermia within two hours of prolonged ischemia significantly protects the BBB [ACTA NEUROPATHOLOGICA; Preston,E; 108(5):406-412 (2004)]. Magnesium can also protect the BBB [JOURNAL OF NEUROSURGICAL ANESTHESIOLOGY; Esen,F; 15(2):119-125 (2003)]. Cryoprotectants typically have high hydrogen bonding capability, but because they are small molecules they do (slowly) cross the BBB. In cryonics patients the BBB is often damaged by ischemic-reperfusion injury, Ironically, the cryoprotectant DMSO is one of several substances (along with ethanol & detergents) that have been used to assist in drug delivery by BBB opening [NEURORX; Pardridge,WM; 2(1):3-14 (2005)]. The phytochemical capsaicin [BRITISH JOURNAL OF PHARMACOLOGY; Hu,D; 146(4):576-584 (2005)] and the contrast agent Optison [ANESTHESIA & ANALGESIA; Mychaskiw,G; 91(4):798-803 (2000)] have also been used to disrupt the BBB.
Although current cryonics protocol achieves enough saturation of the brain with cryoprotectants, the permeation is incomplete, the brain shrinks, with some of the vitrification achieved through dehydration and intracellular proteins. Finding ways to open the blood-brain barrier to allow greater saturation of vitrifying cryoprotectants without causing edema could be a means of increasing ultimate brain viability, provided cryoprotectant toxicity is less damaging than the effects of dehydration.
| Edema in the right leg |
|---|
|
Edema is the swelling of body tissues due to excessive fluid inside cells and/or in the interstitial fluid. Edema is a frequent problem that prevents or limits perfusion of cryonics patients. The patients most often demonstrate a swollen, puffy head or a swollen abdomen. The tissue fluid compresses blood vessels preventing the flow of perfusate. As described in section II, ischemia deprives the sodium pump of energy, causing cells to swell due to excessive influx of sodium ions (which carry additional water into the cells). Ischemia and reperfusion injury can increase capillary permeability and thereby increase interstitial fluid. Many cryonics patients have had disease conditions that increase their susceptibility to edema. Inflammatory conditions, for example, release histamine, which increases capillary permeability.
Four categories of cerebral edema have been described: (1) vasogenic (2) cytotoxic (3) osmotic and (4) interstitial. Vasogenic edema is edema attributed to breakdown of the BBB. Cytotoxic edema is edema due to cell swelling. Osmotic edema is attributed to osmotically active agents, notably AVP (anti-diuretic hormone). Interstitial edema is due to exudation of cerebralspinal fluid from the ventricles of the brain. Interstitial edema is the least frequent form of cerebral edema in medicine and cryonics. The term interstitial edema is also the least appropriate because all edema that is not cytotoxic is associated with accumulation of fluid in the interstitial space. Osmotic edema is third in significance for medicine, and a distant third in cryonics compared to vasogenic and cytotoxic cerebral edema. Cytotoxic edema precedes vasogenic edema in stroke victims [ACTA NEUROPATHOLOGICA; Albayrak,S; 94(2):158-163 (1997)].
The sensitivity of the mitochondrial-rich BBB to hypoxia — and resultant increased permeability from hypoxia — is evidenced by the fact that cerebral edema is seen in high altitude sickness [STROKE; Osta, AV; 36(3):557-560 (2005)]. High capillary pressure or (more often) brain concussion are frequent causes of brain edema in conventional medicine. Leakage of fluid into the brain compresses blood vessels, reducing blood flow and increasing pressure — resulting in a viscious cycle. Oxygen reduction damages capillaries and reduces sodium pump activity, resulting in cell swelling — another viscious cycle. When cerebral blood flow drops below 10-15 milliliters per 100 grams of brain tissue, grey matter begins taking-up water even before the BBB is disrupted [RADIOLOGY; Dzialowsky,I; 243(3):720-726 (2007)].
Four to six hours of brain ischemia leads to a breakdown of the BBB, but in the early hours of ischemia active transport of sodium (along with chloride and water) into the brain is induced by hypoxia, particularly in the luminal membrane — initially causing brain edema, and later causing swelling of BBB endothelial cells [AMERICAN JOURNAL OF PHYSIOLOGY; Brillault,J; 294(1):C88-C96 (2008)]. Reperfusion within an hour of normothermic ischemia in a stroke victim can prevent damage to the BBB, but reperfusion after more than two hours invariably leads to vasogenic edema in one to two days [STROKE; Neumann-Haefelin,T; 31(8):1965-1973 (2000)]. Clinically, stroke victims typically take 2 to 5 days to manifest edema [JOURNAL OF CLINICAL NEUROSCIENCE; Ayata,C; 9(2):113-124 (2002)]. Erythrocyte lysis may be a significant contributor to vasogenic edema, at least in part because iron from hemoglobin breakdown is a potent catalyst of lipid peroxidation [STROKE; Xi,G; 32(12):2932-2938 (2001)]. Superoxide free radicals generated by NADPH oxidase also appears to be a primary contributor to BBB disruption in ischemia/reperfusion [STROKE; Kahles,T; 38(11):3000-3006 (2007)]. Removal of blood cells (leukocytes and erthrocytes) can protect against subsequent reperfusion injury [STROKE; Ding,Y; 33(10):2492-2498 (2002)].
Cytotoxic edema in the brain is primarily due to astrocytes and dendrites rather than neuronal cell bodies [JOURNAL OF NEUROPATHOLOGY AND EXPERIMENTAL NEUROLOGY; Rosenblum,WI; 66(9):771-778 (2007)]. Cell swelling is can be due to reduced ability of energy-depleted cells to pump sodium ions (Na+) out, although there are a number of other mechanisms for astrocyte swelling in cerebral edema [GLIA; Kimbelberg,HK; 50(4):389-397 (2005)]. Sodium ions attract water molecules more strongly than potassium ions, so when sodium ions enter cells they bring more water along. If sodium is able to cross an ischemically damaged BBB it could enter ATP-deficient brain cells and cause cytotoxic swelling. Cytotoxic edema reportedly reduces blood flow much more than vasogenic edema [STROKE; Hossman,KA; 12(2):211-217 (1981)]. Even if sodium does not cross the BBB, it can cause swelling of ATP-deficient endothelial cells and thereby reduce capillary flow in the brain. All cryonics patients — even those who have received prompt stabilization after pronouncement of death — would be expected to have experienced cytotoxic edema. When the BBB is intact, cerebral capillary narrowing due to swollen endothelial cells can be rectified by a hypoosmotic saline/dextran solution [CIRCULATORY SHOCK; Mazzoni,MC; 31(4):407-418 (1990)]. A mild (2ºC) hypothermia has been shown to prevent cytotoxic edema resulting from a moderate decrease in cerebral blood flow in rats [ACTA NEUROBIOLOGIAE EXPERIMENTALIS; Schwab,M; 58(1):29-35 (1998)].
Cellular ATP declines rapidly with ischemia, and there is adequate sodium in the extracellular space to cause cell swelling. Cell swelling by itself does not cause brain edema because brain water, Na+ and Cl− is simply shifted into cells from the extracellular space into cells. But the displacement of these molecules from the extracellular space creates a gradient for drawing them across the BBB into the brain, resulting in brain swelling [LANCET NEUROLOGY; Simard,JM; 6(3):258-268 (2007)]. Hypertonic saline could add to this problem, although that is unproven.
Burr holes are used in medicine to reduce possible brain damage from high IntraCranial Pressure (ICP) resulting from a subdural hematoma — with continuous drainage being more effective than one-time drainage [MEDICAL SCIENCE MONITOR; Kiymaz,N; 13(5):CR240-CR243 (2007)]. Cave paintings and prehistoric skulls indicate that drilling or scraping a hole in the skull to relieve pressure from bleeding associated with blows to the head may have been the earliest surgical procedure for which evidence exists. ICP exceeding 40 mmHg to 50 mmHg can squeeze blood vessels and reduce cerebral perfusion leading to loss of consciousness. Burr holes can protect a cryonics patient from brain damaging ICP due to edema during perfusion.
The alcohol sugar mannitol is frequently used in cryonics and pharmacology
because of its size and properties. Just as glucose can be
reduced to the
sugar alcohol
sorbitol, fructose can be reduced to the sugar alcohol mannitol. Sorbitol and
mannitol have been used as artificial sweeteners because they are minimally absorbed
from the intestine. Even when sorbitol is absorbed it provides only two-thirds the
calories of glucose.
| Reduction of Glucose to Sorbitol | Fructose | Mannitol |
|---|---|---|
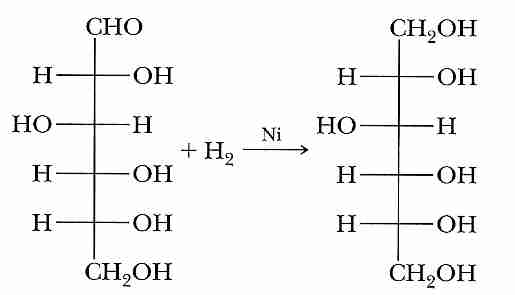
|
|
|
Because mannitol does not cross the BBB it has been used in cryonics to prevent brain edema. Mannitol can cross capillary membranes elsewhere in the body, but not in the brain. Thus, mannitol is an oncotic agent for the brain, but not for the rest of the body. (Mannitol is the M of MHP−2, which has been used as an organ preservation solution by the cryonics organization Alcor.) Raising plasma osmolality from 310 mOsm/kgH2O to 344 mOsm/kgH2O can shrink the brain by 10%, with half of the shrinkage occurring in 12 minutes.
Hyperosmotic (1.5 Molar) mannitol has been used to temporarily open the BBB, allowing for drug delivery. Perfusion of mannitol into the carotid artery shrinks the capillary endothelial cells, thereby widening the tight junctions between the cells for several hours [AMERICAN JOURNAL OF PHYSIOLOGY; Rapoport,SI; 238(5):R421-R431 (1980)]. In an experiment with rats, a 25% mannitol solution infused into the carotid artery for 30 seconds at a rate of 0.25 milliliters/kilogram/second increased BBB transfer of α−aminoisobutyric acid fivefold [JOURNAL OF CEREBRAL BLOOD FLOW & METABOLISM; Chi,OZ; 16(2):327-333 (1996)].
Mannitol has historically been the agent of choice for hyperosmotic treatment of cerebral edema in medicine, but there has been an increasing trend toward the use of hypertonic saline, which reduces neutrophil adherence to endothelial cells [ANNALS OF SURGERY; Pascual,JL; 236(5):634-642 (2002)]. Mannitol, on the other hand, reduces blood viscosity [SURGICAL NEUROLOGY; Andrews,RJ; 39(3):218-222 (1993)] and oxidative damage [BRAIN RESEARCH; 1164:132-135 (2007)]. For cryonics purposes, however, mannitol has the disadvantage of reduced solubility at lower temperature.
Agents used for hyperosmolar treatment of vasogenic edema are assigned reflection coefficients as a measure of the extent that they are reflected-from rather than pass-through the BBB. Sodium chloride is assigned a reflection coefficient of 1.0 to indicate that it does not cross the BBB at all, but is completely "reflected". Mannitol is 90% reflected, and thus has a reflection coefficient of 0.9. Glycerol, with a reflection coefficient of about 0.5 presents the greatest danger that if used for extended medical hyperosmotic treatment it would cross the BBB and begin holding water within the brain [JOURNAL OF NEUROLOGICAL SCIENCES; Ziai,WC; 261(1-2):157-166 (2007)]. Concentrations of glycerol in excess of 20% can cause hemolysis [ACTA NEUROCHIRUGICA; Biestro,A; 139(8):725-733 (1997)]. An initial bolus of mannitol transiently dehydrates the brain, but repeated doses exacerbates edema [JOURNAL OF NEUROSURGERY; Kaufmann,AM; 77(4):584-589 (1992)]. The BBB actively extrudes sodium, so it has less potential for a rebound effect than mannitol in patients who are metabolically active (rather than ischemic cryonics patients) [JOURNAL OF NEUROSURGICAL ANAESTHESIOLOGY; Qureshi,AI; 10(3):188-192 (1998)].
| ICP lowering by hypertonic saline and mannitol |
|---|
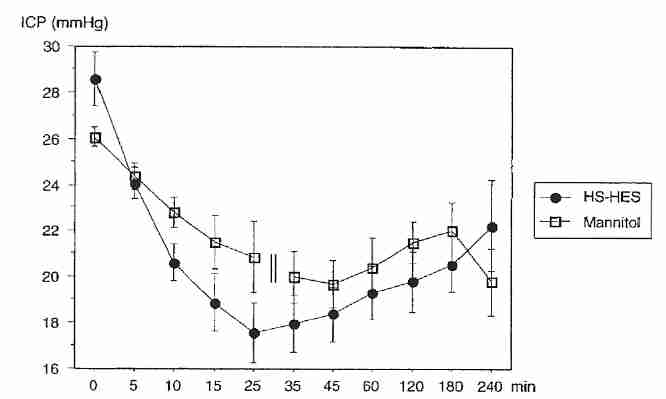
|
Rat experiments with equimolar hypertonic saline (7.5%) or mannitol (20%) used for hypertonic treatment of experimental stroke have generally shown hypertonic saline to be superior in reducing brain water content [CRITICAL CARE MEDICINE; Toung,TJ; 33(1):203-208 (2005)]. Similar results have been seen with clinical trials comparing IntraCranial Pressure (ICP) in human stroke patients treated with equimolar hypertonic saline (7.5%) or mannitol (20%) [ANESTHESIOLOGY; Rozet,I; 107(5):697-704 (2007) and STROKE; Schwarz,S; 29(8):1550-1555 (1998)]. Although 20% mannitol and 7.5% saline are equimolar, 20% mannitol is equiosmolar with 3.6% saline.
Using equimolar solutions of mannitol and saline makes for good direct comparison, but does not answer the question of what would be the optimal concentration of either mannitol or saline. And what is optimal for medicine might not be optimal for cryonics. In medicine, care is taken to prevent hemolysis, but this might not be such a worry for cryonics insofar as the blood is being washed-out. Shrinkage of red blood cells by hypertonic dehydration can be beneficial in helping to remove cells, except for the fact that dehydrated red blood cells have increased adhesion to the walls of blood vessels [BRITISH JOURNAL OF HAEMATOLOGY; Wandersee,NJ; 131(3):366-377 (2005)].
Another possible complication is the fact that mannitol is used clinically to open the BBB to allow drugs to enter the brain [CANCER TREATMENT REVIEWS; Kemper,EM; 30(5):415-423 (2004)]. Water influx into the brain following BBB opening by mannitol is desirable for the uptake of drugs, but would only be desirable in cryonics if cryoprotectant uptake was inhanced — but water replacement by cryoprotectant is the goal. Five minutes of 1.6 molar mannitol is adequate to open the BBB [BRAIN RESEARCH PROTOCOLS; Bhattacharijee,AK; 8(2):126-131 (2001)], but 1.1 molar mannitol is adequate at 4ºC because hypothermia helps mannitol to open the BBB [BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS; Ikeda,M; 291(3):669-674 (2002)]. Nonetheless, mannitol is not very water soluble: 1.66 molar mannitol can precipitate at 37ºC, which is why the more soluble sugar arabinose has been used instead [AMERICAN JOURNAL OF PHYSIOLOGY; Rapoport,SI; 239(5):R421-R431 (1980)].
21st Century Medicine (21CM) has systematically screened molecules for inhibition of cell swelling, and found alpha lactose to be especially effective. 21CM patented an organ preservation solution they call TranSend, which is based on alpha lactose and polyglycerol. No mention is made about whether alpha lactose crosses the BBB. Although lactose is less expensive than mannitol, it has a larger molar mass (342 versus 182 for mannitol) and thus would be expected to be less effective as an osmotic agent and more viscous on a per gram basis. To the extent that mannitol does not cross the BBB, it will nonetheless reduce cells swelling in the brain simply by dehydrating the brain. Thus, mannitol is often the agent of choose for hyperosmotic blood washout in cryonics, although its poor solubility characteristics (especially at low temperature) can present problems.
(For more on ischemia/reperfusion damage to the blood-brain barrier, see Reperfusion Injury and "No Reflow".)
Dextran is a glucose polysaccharide with molecular mass designated in kilodaltons, e.g., Dextran−60 has a molecular weight of 60,000 (60 kilodaltons). (Plasma is about 4-5% albumin, and albumin has a molecular weight of about 65,000.) Dextran reduces adherence of leukocytes and platelets to endothelial cells during reperfusion, with increasingly large molecular weights increasingly reducing adhesion [AMERICAN JOURNAL OF PHYSIOLOGY; Steinbauer,M; 272(4 Pt 2):H1710-H1716 (1997)]. Dextrans also enhance fibinolysis [CRITICAL CARE MEDICINE; de Jonge,E; 29(6):1261-1267 (2001)].
Capillary flow after hemorrhagic shock can be impeded by endothelial cell swelling (a type of cytotoxic edema). A mixture of 7.2% NaCl and 10% dextran−60 has been shown to significantly reduce endothelial cell swelling after hemorrhagic shock [JOURNAL OF SURGICAL RESEARCH; Corso,CO; 80(2):210-220 (1998)]. A 7.2% NaCl/10% dextran−60 solution is hyperosmotic and hyperoncotic relative to plasma. The narrowed capillaries may be plugged by leucocytes, leading to "no reflow" on reperfusion, rectified by hyperosmotic/hyperoncotic NaCl/dextran solution [CIRCULATORY SHOCK; Mazzoni,MC; 31(4):407-418 (1990)]. High viscosity of the resuscitation fluid may be more important than being hyperoncotic [SHOCK; Cabrales,P; 22(5):431-437 (2004)].
The BBB becomes increasingly leaky with progressive ischemia/reperfusion time (vasogenic edema), but increasingly large dextrans can be used to maintain oncotic pressure [MICROCIRCULATION; Nagaraja,TN; 15(1):1-15 (2008)]. But even beyond the point where large dextrans begin leaking across the BBB (21 hours after a 3-hour ischemic episode) and are no longer capable of maintaining oncotic pressure, they can benefit perfusion in an ischemic cryonics patient by clearing leucocytes and platelets from blood vessels.