by Ben Best
[For background on the chemistry and biochemistry of free radicals — and antioxidant enzymes — see The Free Radical Theory of Aging.]
Free radicals are molecules containing unpaired electrons. The unpaired electron is a highly reactive "hot potato" that either "burns" a molecule (causes oxidative damage) or is passed from molecule to molecule causing turning the recipient into a free radical and neutralizing the donor. A more accurate metaphor, however, is to describe an unpaired electron as a molecular "shark" that seizes an electron from another molecule, leaving the "victim" molecule with an unpaired electron. (The "hot potato" is literally the absence of an electron partner for an unpaired electron.) Most often the unpaired electron ("shark") will seize a hydrogen atom (which is as good or better than an electron, insofar as hydrogen atoms do not hold electrons very strongly) from another molecule. An example would be the case of the hydroxyl radical (.OH) seizing a hydrogen atom from a reduced glutathione (GSH) molecule, resulting in a water molecule and a glutathione radical:
.OH + GSH —> H2O + GS.
In the case of lipid peroxidation, there is a chain reaction which involves both damage and passing of radicals. Cellular macromolecules are vulnerable to free radical damage: lipids, protein and nucleic acids can all be damaged.
Free radical damage to LDL cholesterol leads to atherosclerosis, so antioxidants have the potential to protect against cardiovascular disease. Similarly, free radicals have been implicated in cancer, Alzheimer's Disease, inflammatory diseases, ischemic-reperfusion injury and a myriad of other disease conditions against which antioxidants may be of benefit.
According to the Free Radical Theory of Aging free radicals may even underlie the aging process itself, accounting for why mice age so much more rapidly than humans. Most of the free radicals are produced by mitochondria, and most of the free radical damage is to mitochondrial membranes and mitochondrial DNA [EXPERIMENTAL BIOLOGY AND MEDICINE; Wei, YH; 227:671-682 (2002)]. Between one and five percent of the oxygen used by mitochondria to generate energy results in the formation of superoxide radicals. Nonetheless, the most long-lived species have the lowest levels of antioxidant enzymes — having low rates of free radical production and high efficiency of DNA repair [JOURNAL OF COMPARATIVE PHYSIOLOGY B; Perez-Campo,R; 168(3):149-158 (1998)].
Most free radicals in biological systems are derivatives of oxygen ("Reactive Oxygen Species", ROS), but there are also derivatives of nitrogen ("Reactive Nitrogen Species", RNS). Conditions of high prooxidant activity due to free radicals are often described by the phrase oxidative stress, however. The most reactive and damaging free radicals are the hydroxyl radical (especially) and the peroxynitrite radical.
Although mitochondria are the major source of free radicals, there are numerous other sources. The inflammatory cytokine Tumor Necrosis Factor−alpha (TNF−α) stimulates free radical production by mitochondria. Ultraviolet light (UV) produces free radicals. Free radicals are released from white blood cells (neutrophils) associated with inflammation. Neutrophils use oxidative free radicals (superoxide, hydrogen peroxide, hydroxyl) to kill bacteria. The lysosomal enzyme myeloperoxidase catalyzes the production of bacteriocidal hypochlorite from hydrogen peroxide and chloride ions. Free radicals are generated by eicosanoids from arachidonic acid during ischemia-reperfusion injuries. During reperfusion the endothelial enzyme xanthine oxidase converts oxygen to superoxide, which can react with nitric oxide to produce peroxynitrite. Free radicals from tobacco smoke and air pollution can cause oxidative damage to lungs, blood vessels and other body tissues.
Antioxidants are molecules that can neutralize free radicals by accepting or donating an electron to eliminate the unpaired condition. Typically this means that the antioxidant molecule becomes a free radical in the process of neutralizing a free radical molecule to a non-free-radical molecule. But the antioxidant molecule will usually be a much less reactive free radical than the free radical neutralized. The antioxidant molecule may be very large (allowing it to "dilute" the unpaired electron), it may be readily neutralized by another antioxidant and/or it may have another mechanism for terminating its free radical condition.
Molecules with loosely-held hydrogen atoms can use those hydrogen atoms like electrons to neutralize free radicals. The hydrogen atoms are called reducing equivalents, and the molecules having such hydrogen atoms are said to be in a reduced state.

|
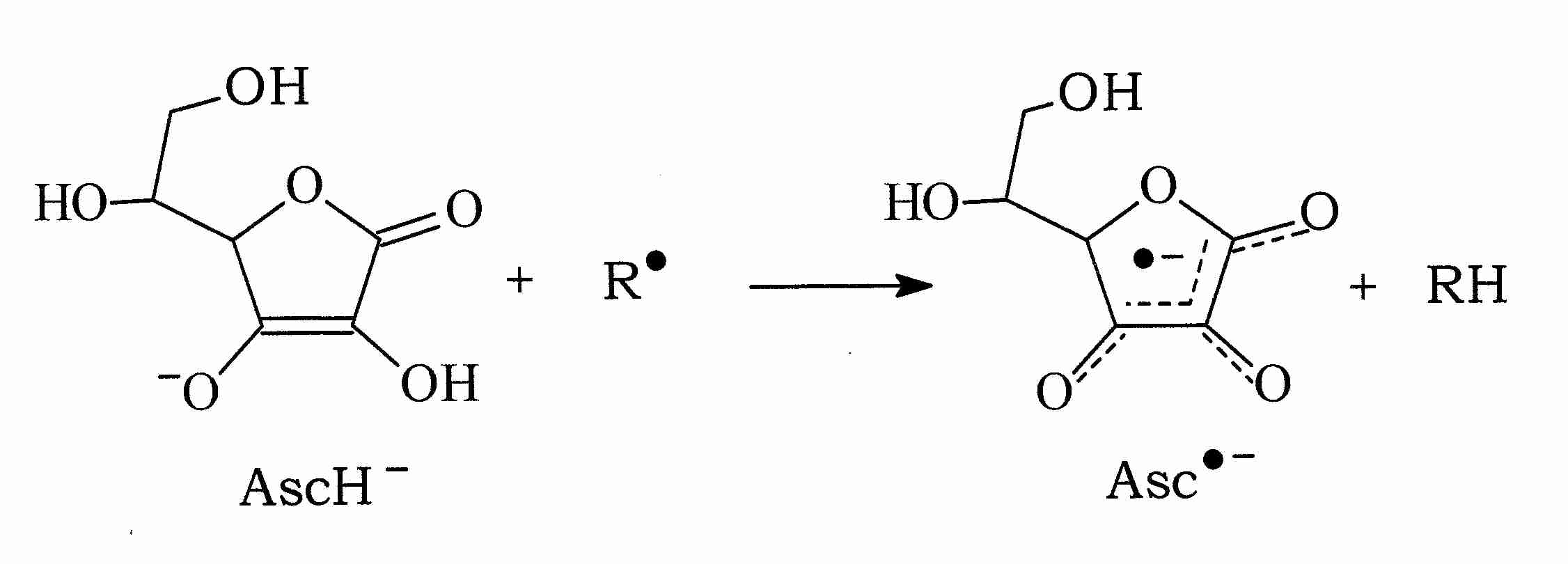
Vitamin C (ascorbate, AscH-), for example, can donate a hydrogen atom to a free radical molecule (R.) thereby neutralizing the free radical while becoming an ascorbate radical itself (.Asc-, or Asc.-, in different notation). But the .Asc- free radical is very stable because of its resonance structure (shown by the dashed lines in the illustration). Moreover, AscH- is readily regenerated from .Asc- with NADH or NADPH-dependent reductases [THE JOURNAL OF BIOLOGICAL CHEMISTRY; Hossain, MA; 260(24):12920-12926 (1985)].
Vitamin C can not only neutralize hydroxyl (.OH), alkoxyl (.OL) and peroxyl (LOO.) radicals by hydrogen donation, ascorbate can also neutralize the radical form of other antioxidants, such as glutathione (.GS) and Vitamin E (tocopherol) (.Toc):
|
AscH- + .Toc —> Toc + .Asc-
Free radicals can be listed by one-electron reduction potentials in milliVolts (mV) at pH 7.0. The reduced form of each radical is capable of neutralizing (reducing) free radicals having a higher potential. As can be seen from the table, the hydroxyl radical (.OH) has the highest potential and is the most destructive (reactive) of biological free radicals. (Source [RADIATION RESEARCH; Buettner, GR; 145:532-541 (1996)].)
Chelation of Fe3+ with EDTA actually enhances the reactivity of iron toward superoxide, thus favoring the Haber-Weiss Reaction.
Fe3+-EDTA chelate can catalyze the Fenton Reaction to generate hydroxyl ion without reduction of Fe3+ to Fe2+. On the other hand, Fe3+ and Fe3+-EDTA can be reduced to Fe2+ by ascorbate (AscH-) to generate the ascorbate radical (.Asc-). The reduced iron can then generate a hydroxyl radical by the Fenton Reaction. Copper ion (Cu2+) is 80 times more efficient at reacting with ascorbate than Fe3+. Thus, Vitamin C can be a powerful antioxidant as long as metal ions are not present, but small amounts of Vitamin C in the presence of metal ions can make Vitamin C a dangerous prooxidant. Large amounts of Vitamin C can restore the antioxidant function. (Vitamin C has been called an "oxymoron antioxidant"). For an excellent review on this subject, see [RADIATION RESEARCH; Buettner, GR; 145:532-541 (1996)].
The reactions in which the metal ion is regenerated as a catalyst can be summarized as:
Fe++ + H2O2 —> Fe+++ + .OH + :OH-
Fe+++ + AscH- —> Fe++ + .Asc- + H+
Normally iron and copper are tightly bound in the body to carrier proteins (ferritin, transferritin and cearuloplasmin), but the introduction of metal ions from tobacco smoke and other pollutants into the bloodstream can circumvent this protection. Copper differs from iron, however, by producing more singlet oxygen than hydroxyl radical when reacted with H2O2 [CHEMICAL RESEARCH IN TOXICOLOGY; Frelon,S; 16(2):191-197 (2003)]. And singlet oxygen reacts with DNA to produce 8−OHdG [JOURNAL OF BIOLOGICAL CHEMISTRY; Ravanat,J; 275(51):40601-50604 (2000)].
Primates have lost the capacity to synthesize ascorbate, and therefore have a requirement for the substance as a vitamin (Vitamin C) in the diet. But primates compensate for this loss by having very high serum levels of the water-soluble anti-oxidant urate (uric acid). An analysis of mammalian species has shown a significant positive correlation between urate per specific metabolic rate (calories per gram per day) and maximum lifespan [ARCHIVES OF GERONTOLOGY AND GERIATRICS; Cutler,RG; 3:321-348 (1984)]. Uric acid is not only a water-soluble antioxidant and singlet oxygen quencher, but is an iron chelator that can protect ascorbate from iron-catalyzed generation of reduced iron [AMERICAN JOURNAL OF CLINICAL NUTRITION; Sevanaian,A; 54:1129S-1134S (1991)].
Thiols (molecules with sulfhydryl [ −SH ] groups) such as thioredoxins (small disulphide-containing proteins), cysteine and reduced glutathione (GSH) are also examples of molecules that can reduce free radicals by hydrogen donation. Metallothioneins are small cysteine-rich proteins that can reduce oxidative stress by metal-binding as well as by hydrogen donation [MOLECULAR AND CELLULAR BIOLOGY; Zhang,B; 23(23):8471-8485 (2003)]. Reduced glutathione hydrogen donation from the sulfhydryl group of cysteine can neutralize a hydroxyl radical or a Vitamin C radical:
GSH + .OH —> .GS + H2O OR GSH + .Asc- —> .GS + AscH-
and then oxidized glutathione radicals can neutralize each other:
.GH + .GH —> GSSG
The enzyme glutathione peroxidase utilizes reduced glutathione to eliminate hydrogen peroxide:
2 GSH + H2O2 —> GSSG + 2 H2O
Glutathione reductase then adds hydrogens to the oxidized glutathione (GSSG) to regenerate reduced glutathione (GSH). A high GSH/GSSG ratio indicates a high level of reduced glutathione available for antioxidant activity. Normal mouse liver GSH/GSSG ratios typically range from 50 to 200. The ratio of oxidized glutathione (GSSG) to total glutathione (GSSG + GSH) in human blood for a person in their sixties is more than double that seen in a teenager [AMERICAN JOURNAL OF CLINICAL NUTRITION; Sen,CK; 72(Suppl):653S-650S (2000)].
Glutathione peroxidase contains selenium. Selenium in the diet can increase glutathione peroxidase levels, which is why a Recommended Dietary Allowance (RDA) for selenium was established in the United States in 1989. Selenium deficiencies result in glutathione peroxidase depletion. Epidemiological studies strongly indicate a protective effect of selenium against many cancers. Selenium can also reduce mercury toxicity, but in excess selenium is a toxic poison itself.
There are many similar biochemical processes that oxidize reduced antioxidant molecules to neutralize free radicals and then restore the antioxidant molecules to a reduced state. In mitochondrial membranes, Vitamin E that has donated a hydrogen to neutralize a free radical can be regenerated (reduced) by CoEnzyme Q, which has two hydrogens to donate, and can avoid becoming a free radical by donating both hydrogens.
Organisms possess natural defenses against free radicals in the form of antioxidant enzymes, such as superoxide dismutase (which neutralizes superoxide) and catalase (which neutralizes hydrogen peroxide). Organisms also synthesize non-enzymatic antioxidant molecules such as glutathione and CoEnzyme Q. Animals can also obtain antioxidants through diet, such as Vitamin E and the phytochemical carotenoid substances. The element selenium has antioxidant properties because it is an essential component of the enzyme glutathione peroxidase — which uses glutathione to neutralize hydrogen peroxide. Metal chelating substances can be antioxidant by preventing metal ions from producing free radical reactions.
The most effective singlet oxygen quenchers are carotenoids, phytochemicals which plants produce to protect themselves from singlet oxygen produced by ultraviolet light. Singlet oxygen is not a free radical, but it has electrons in an excited state that react destructively with biomolecules containing double-bonds. Carotenoids neutralize singlet oxygen almost entirely by physical quenching, a process in which singlet oxygen is restored to its ground state with no oxygen consumption or product formation (in contrast to chemical quenching which results in a dioxide) [ARCHIVES OF BIOCHEMISTRY AND BIOPHYSICS; Stahl,W; 336(1):1-9 (1996)]. A single beta-carotene molecule can quench 250 to 1000 singlet oxygen molecules at a rate of 14 X 10−9 M−1S−1 and lycopene can quench at over twice that rate [ARCHIVES OF BIOCHEMISTRY AND BIOPHYSICS; Di Mascio,P; 274(2):532-538 (1989)]. (The second order rate constant — ie, squared concentration-dependent rate constant — is expressed in M−1S−1 , where M = Molarity = moles/liter and S = Seconds.)
Epidemiological studies have shown increased beta-carotene consumption and elevated beta-carotene levels in blood plasma were associated with decreased risk of lung cancer. So it was reasonable to assume that beta-carotene supplementation might reduce the incidence of lung cancer. Surprisingly, large intervention studies showed increased lung cancer rates with beta-carotene supplementation in smokers, with the worst increase associated with the heaviest smoking and higher alcohol intake [JOURNAL OF THE NATIONAL CANCER INSTITUTE; Albanes,D; 88(21):1560-1565 (1996)]. Vitamin C, which can facilitate Vitamin E recycling and beta-carotene stability, was not used in the studies.
Serum levels of beta-carotene in those supplemented group were over four times than normally found in those getting beta-carotene from vegetables. Animal studies suggest that abnormal carotenoid products formed in the liver (possibly worsened by alcohol-induced enzyme dysfunction) could have led to the destruction of Vitamin A and the exacerbation of lung cell proliferation [JOURNAL OF NUTRITION; Russell,RM; 134:263S-268S (2004)].
A multiplicity of antioxidants is beneficial because specific antioxidant molecules can be particularly effective for neutralizing specific ROS or RNS. For example, gamma-tocopherol is particularly effective against the peroxynitrite radical. A multiplicity of antioxidants is also beneficial because different antioxidants tend to locate preferentially in different areas of tissues and cells. Melatonin is a potent inhibitor of hydroxyl radicals, and it readily crosses cell membranes and the blood-brain barrier — making it particularly effective for protecting DNA in the nucleus & mitochondria of the brain. The most powerful carotenoid is lycopene, which has 100-times the singlet-oxygen quenching action of Vitamin E, which (in turn) has 125-times the quenching action of glutathione. But lycopene & Vitamin E are only effective in lipid-containing areas, whereas glutathione can be found in the "watery" areas.
Although Vitamin E (tocopherol, Toc) is only moderately effective against singlet oxygen, it is the most effective antioxidant for terminating the chain reactions of lipid peroxidation in cell membranes.
.L (or LO. or LOO.) + TocH —> LH (or LOH or LOOH) + Toc.
But tocopherol must be regenerated (ie, reduced by addition of hydrogen atoms) by other antioxidants. The tocopherol radical (Toc.) is at best useless for further lipid peroxidation termination, and at worst a pro-oxidant. It must be regenerated (recycled) to the reduced form by other antioxidants. Smokers given a high polyunsaturated diet (safflower oil) suffer oxidative damage that is considerably worsened by the addition of alpha-tocopherol [ARTERIOSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY; Weinberg,RB; 21(6):1029-1033 (2001)].
|
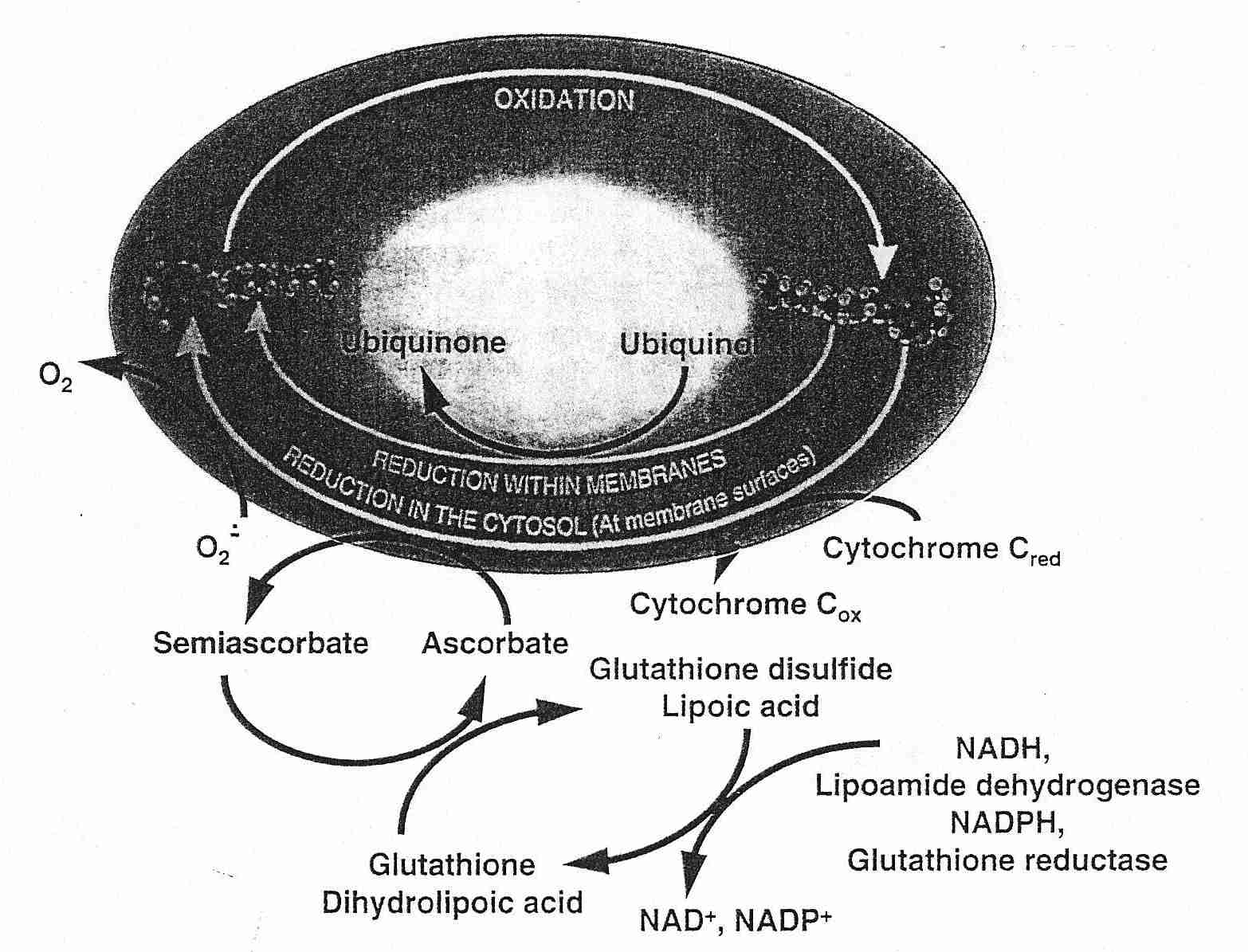
CoEnzyme Q (ubiquinol) or Vitamin C regenerates Vitamin E — and Vitamin C is in turn regenerated by glutathione or lipoic acid. Glutathione is effective against hydrogen peroxide and hydroxyl radicals in the aqueous phase of cells. Lipoic acid is more effective than glutathione in regenerating Vitamin C and can additionally chelate free-radical-generating metal ions. NADH or NADPH (energy-containing molecules resulting from glycolysis or oxidative phosphorylation) can regenerate glutathione or lipoic acid, thus completing the antioxidant network for membrane lipid peroxidation.
It is easy to imagine that a necessary condition for being able to regenerate all intracellular antioxidant molecules to a reduced (rather than oxidized, pro-oxidant) form is by having high levels of reducing equivalents (NADH, NADPH) in the cell. This may be true, but it is also true that a high NADH/NAD+ ratio leads to hydrogen peroxide (ROS) production by the citric acid cycle enzyme complex alpha-ketoglutarate dehydrogenase [THE JOURNAL OF NEUROSCIENCE; Tretter,L; 24(36):7771-7778 (2004)].
Antioxidants are notable for boosting the immune system because immune system cells in the bloodstream are so easily accessed by free radicals as well as by antioxidants. Cells of the immune system (like T-Cells, B-Cells and Macrophages) have membranes that are particularly rich in long-chain unsaturated fatty acids (such as arachidonic acid or EPA). For this reason (and also because of their mobility and functions) immune system cells are more vulnerable to free radical oxidation than other cells. The nutrients that most profoundly improve immune function are Vitamin C, Vitamin E, selenium, glutathione and zinc [AMERICAN JOURNAL OF CLINICAL NUTRITION; Meydani, SN; 52:557-563 (1990)]. (Whey protein and N-Acetyl Cysteine both boost glutathione levels.)
All of these nutrients are antioxidants, although zinc's effects are more due to direct actions on immune cell function than due to its antioxidant properties [AMERICAN JOURNAL OF CLINICAL NUTRITION; Shankar, A; 68(Supp):447S-463S (1997)]. Vitamin E opposes some, but not all of the increased lipid peroxidation and immune suppression seen in essential fatty acid supplementation [AMERICAN JOURNAL OF CLINICAL NUTRITION; Kramer, TR; 54:896-902 (1991), LIPIDS;Fernandes, G; 31:S91-S96 (1996) and LIPIDS 32:535-541 (1997)]. Independent of its antioxidant effect, vitamin E promotes immune function by reducing PGE2 synthesis. Vitamin E directly opposes the increase in PGE2 formation that is typically seen in aging. PGE2 is known to suppress lymphocyte proliferation, to suppress synthesis of chemical factors (lymphokines) influencing the immune system and to contribute to the auto-immune diseases that increase with aging [AMERICAN JOURNAL OF CLINICAL NUTRITION; Meydani, SN; 62(Supp):1462S-1476S (1995) and NUTRITION REVIEWS; Meydani, SN; 53(4):S52-S58 (1995)].
Natural antioxidant enzymes manufactured in the body provide an important defense against free radicals. Glutathione peroxidase, glutathione reductase, catalase, thioredoxin reductase, superoxide dismutase, heme oxygenase, methionine sulfoxide reductase, and biliverdin reductase, are the most important antioxidant enzymes. The enzyme superoxide dismutase converts two superoxide radicals into one hydrogen peroxide and one oxygen. To eliminate hydrogen peroxide before the Fenton Reaction can create a hydroxyl radical, organisms use catalase and/or glutathione peroxidase. The brain, which is very vulnerable to free radical damage (due to high metabolic rate, high unsaturated fat in neurons, and the fact that neurons are post-mitotic) has seven times more glutathione peroxidase activity than catalase activity [CANCER RESEARCH; Marklund,SL; 42(5):1955-1961 (1982)]. Moreover, glutathione peroxidase is found throughout the cell, whereas catalase is often restricted to peroxisomes. Nonetheless, the lifespan of transgenic mice has been extended about 20% by overexpression of human catalase targeted to mitochondria [SCIENCE; Schriner,SE; 308:1909-1911 (2005)].
The Superoxide dismutase (SOD) molecule in the cytoplasm contains copper & zinc atoms (Cu/Zn−SOD), whereas the SOD in mitochondria contains manganese (Mn−SOD). Unlike exogenous antioxidants, which are generally depleted by antioxidant action, antioxidant enaymes are not depleted because they act catalytically. An exogenous orally effective SOD mixture has been shown to protect against hyperbaric oxidation damage to DNA that cannot be prevented with Vitamin E or N-Acetyl Cysteine [FREE RADICAL RESEARCH; Muth,CM; 38(9):927-932 (2004)].
Superoxide dismutase without glutathione peroxidase or catalase (CAT) to remove hydrogen peroxide is of little value. Insects lack glutathione peroxidase, but experiments have been performed on fruit flies made transgenic by having extra genes for SOD, CAT or both. The flies that were given extra genes for SOD or CAT (but not both) had no more than a 10% increase in mean lifespan, with no increase in maximum lifespan. But flies that had extra genes for both SOD and CAT showed maximum lifespan increase by as much as a third, while showing less protein oxidative damage and better physical performance [SCIENCE 263:1128-1130 (1994)]. A similar experiment using SOD/CAT mimetics in nematode worms increased mean lifespan 44% [SCIENCE 289:1567-1569 (2000)]. And selective inbreeding of bread-mold fungus resulted in strains with lifespans more than 6 times longer than wild-type — a change that was shown to be due to increased expression of antioxidant enzymes [FREE RADICAL BIOLOGY & MEDICINE 8:355-361 (1990)]. Females express both more Mn−SOD and more glutathione peroxidase than males, and this has been suggested to be the reason females live longer than males in mammalian species [FEBS LETTERS; Vina,J; 579(12):2541-2545 (2005)]. The lifespan of transgenic mice has been extended about 20% by overexpression of human catalase targeted to mitochondria [SCIENCE; Schriner,SE; 308:1909-1911 (2005)].
The glutathione system (glutathione, glutathione peroxidase and glutathione reductase) is a key defense against hydrogen peroxide and other peroxides. There are four forms of glutathione peroxidase (GPx) enzymes: (1) cystolic Glutathione Peroxidase (cGPx, ubiquitously distributed), (2) Phospholipid Hydroperoxidase Glutathione Peroxidase (PHGPx, in plasma membranes to reduce hydroperoxides of complex lipids), (3) plasma Glutathione Peroxidase (pGPx, in blood plasma) and (4) Gastro-Intestinal Glutathione Peroxidase (GIGPx, in the liver and GI tract only). The kidney manufactures most pGPX. Both pGPX and PHGPx can counteract LDL peroxidation in plasma and endothelial cells. PIGPx probably protects against dietary hydroperoxides. Selenium is an essential component of GPx, so the relative preservation of enzyme levels during selenium deficiency may provide a guide to their relative importance. GPGPx is the most highly preserved:
GIGPx > PHGPx > pGPx = cGPx
The enzymes are not equally preserved in all tissues during selenium deficiency, which may provide a basis for ranking relative importance in different tissues. The tissues most highly conserving cGPx and PHGPx are:
cGPx : brain >> thymus > thyroid > heart > liver,kidney,lung
PHGPx : brain > testes >> heart > liver,kidney,lung
[FREE RADICAL BIOLOGY & MEDICINE; Brigelius-Flohe,R; 27(9/10):951-965 (1999)]
Vitamin C and Vitamin E can compensate for decline in glutathione associated with aging, but excess Vitamin C and Vitamin E apparently reduces glutathione synthesis, thereby weakening cellular defense against oxidative stress [FREE RADICAL BIOLOGY & MEDICINE; Shang,F; 34(5):521-530 (2003)].
The sulfur-containing amino acids methionine and cysteine are the most readily oxidized of any of the amino acids — both as free amino acids or in proteins. Methionine is oxidized to methionine sulfoxide, but methionine sulfoxide reductases enzymatically regenerate methionine [BIOPHYSICA ET BIOCHEMICA ACTA; Lee,BC; 1790 (11): 1471-1477 (2009)]. In turn, the thioredoxin system reduces (recycles or regenerates) some of the methionine sulfoxide reductases [PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES (USA); Sagher,D; 103(23):8656-8661 (2006)].
As with the glutathione system, the thioredoxin system (thioredoxin/thioredoxin reductase) functions to maintain the cellular environment in a reduced state (decreasing disulfide cross-linking) and protects against free radicals. Transgenic mice that overexpress thioredoxin are more resistant to oxidative stress and inflammation — and live about 35% longer [ANNALS OF THE NEW YORK ACADEMY OF SCIENCES; Yoshida,T; 1055:1-12 (2005)]. Thioredoxins are small (12 KiloDalton) proteins with an active dithiol (two −SH groups nearby because of cysteine residues) site: −Cys−Gly−Pro−Cys−. NADPH reduces (donates hydrogen atoms) to regenerate reduced thioredoxin [TRX−(SH)2] just as it does to regenerate reduced glutathione [GSH].
The thioredoxin system for elimination of protein disulfides can be summarized as follows:
TRX−(SH)2 + Protein−S2 -> TRX−S2 + Protein−(SH)2
TRX−S2 +NADPH + H+ -> TRX−(SH)2 (catalyzed by thioredoxin reductase)
Like glutathione peroxidase, thioredoxin reductase is a selenium-containing enzyme whose activity can be influenced by dietary selenium. There are two forms of thioredoxin, TRX1 (104 amino acids, found in the cytoplasm) and TRX2 (found in mitochondria). In addition to its direct antioxidant actions, TRX1 can induce increased transcription of superoxide dismutase [AMERICAN JOURNAL OF RESPIRATORY CELL AND MOLECULAR BIOLOGY; Das,KC; 17(6):713-726 (1997)].
The regulatory gene sequence for producing antioxidant enzymes, both constitutively and by induction, is called the Antioxidant Response Element (ARE). The most important transcription factors for regulating and upregulating the ARE are Nuclear Factor Erythroid 2 p-45 related factors 1 and 2 (especially NF-E2-related factor 2, Nrf2). Nrf1 and Nrf2 are phase 2 detoxification enzyme inducers, which means they also induce enzymes which conjugate toxins to increase water solubility and excretion.
Normally sequestered in the cytoplasm by the inhibitor molecule Keap−1, Nrf2 migrates to the nucleus under conditions of oxidative stress that oxidize Keap−1 cysteine residues. GSH plays a role in causing Nrf2 to migrate to the nucleus and TRX1 creates the reduced environment which is required for Nrf2 to bind to DNA [TOXICOLOGICAL SCIENCES; Hansen,JM; 82(1):308-317 (2004)]. Nrf2 also regulates the activity of glutathione-synthesizing enzyme, both under normal conditions (constitutive gene expression) and under conditions of oxidative stress (which induces more gene expression). With age Nrf2 levels can fall by half, resulting in declining levels of glutathione as well as decreased capacity to increase glutathione synthesis in response to oxidative stress. Lipoic acid has been shown to restore Nrf2 activity in the liver of aging rats [PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES (USA); Suh,JH; 101(10):3381-3386 (2004)].
|
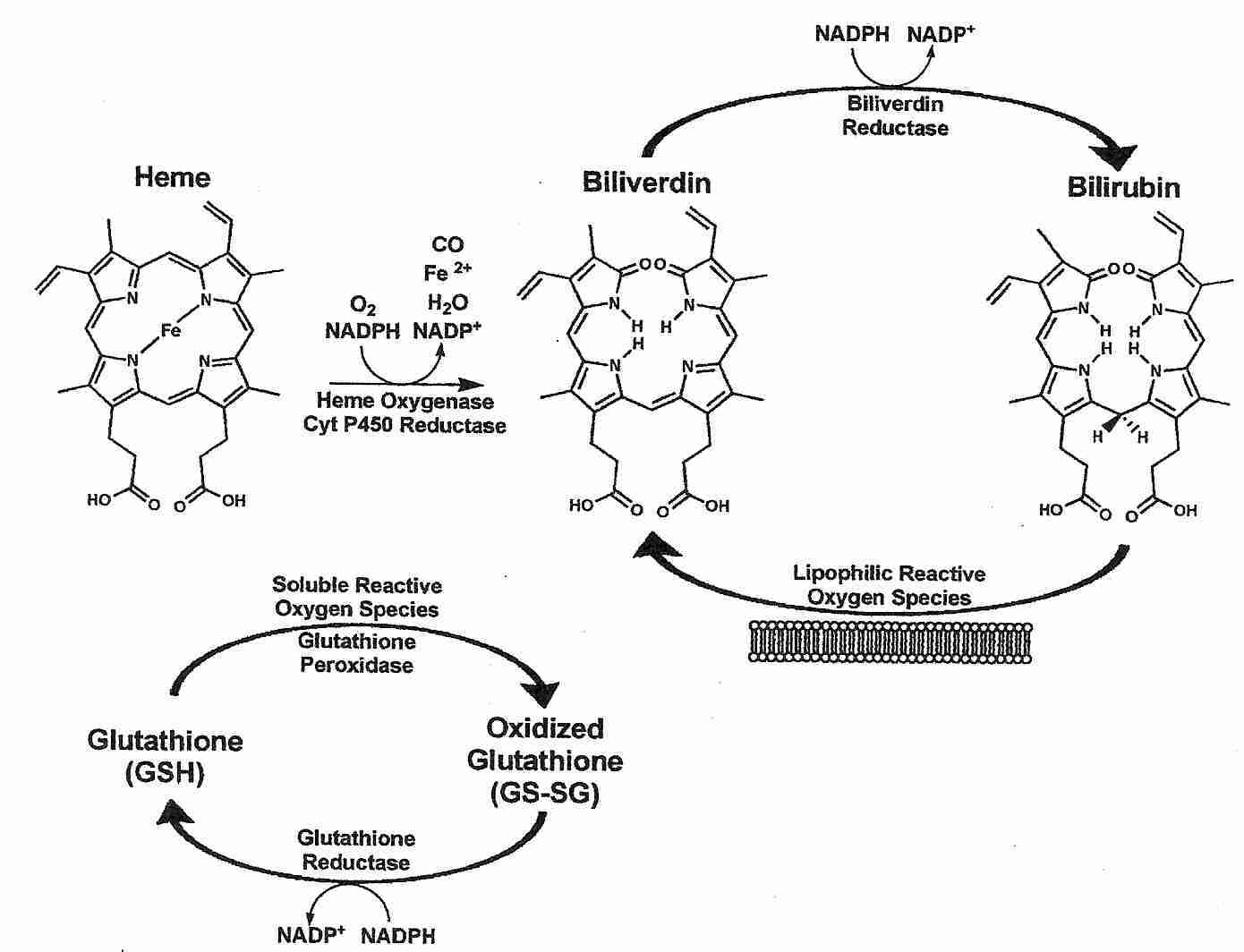
The enzyme heme oxygenase catalyzes the breakdown of heme (the heme of hemoglobin) to the products carbon dioxide, iron and biliverdin. Biliverdin is immediately converted to bilirubin by the enzyme biliverdin reductase. Although production of ferrous (Fe2+) iron could potentially result in free radical production, heme oxygenase activity is tightly coupled to ferritin protein synthesis, which results in binding of iron [THE JOURNAL OF CLINICAL INVESTIGATION; Nath,KA; 90(1):267-270 (1992)].
Bilirubin is a powerful lipophilic antioxidant that protects membranes from lipid peroxidation and protects membrane proteins from oxidation. Much of the power of bilirubin as an antioxidant comes from the extreme rapidity with which biliverdin (oxidized bilirubin) is converted to bilirubin by bilverdin reductase. A small concentration of bilirubin can protect against a 10,000-fold greater concentration of hydrogen peroxide due to the abundance and rapid action of biliverdin reductase in all tissues [PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES (USA); Baranano,DE; 99(25):16093-16098 (2002)]. Bilirubin recycling may be much faster than glutathione recycling at least partially because glutathione recycling requires two enzymes rather than one: glutathione peroxidase and glutathione reductase.
Bilirubin accounts for most of the antioxidant activity of human serum and is particularly potent against superoxide and peroxyl radicals. Risk of atherosclerosis has been shown to vary inversely with serum levels of bilirubin [EXPERIMENTAL BIOLOGY AND MEDICINE; Novotny,L; 228(5);568-571 (2003)]. The Framingham Heart Study found high serum bilirubin correlated with low cardiovascular risk for men [THE AMERICAN JOURNAL OF CARDIOLOGY; Djousse,L; 87(10):1196-1200 (2001)]. A ten-year Belgian study of nearly 10,000 people found high serum bilirubin correlated with low cancer mortality (especially for men), but found no association with cardiovascular disease [CANCER CAUSES AND CONTROL; Temme,EH; 12(10):887-894 (2001)]
There are two forms of Heme Oxygenase (HO) enzyme: (1) HO−1, an inducible heat shock protein which is especially concentrated in the spleen & liver and (2) HO−2 which is a constitutive enzyme especially concentrated in the brain & testes [PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES (USA); Dore,S; 96(5):2445-2450 (1999)]. HO−1 acts as an antioxidant by releasing Fe+2 from heme and thereby inducing expression of the Fe+2-sequestering protein ferritin [THE FASEB JOURNAL; Berberat,PO; 17(12):1724-1747 (2003)]. HO−1 synthesis, as with other anti-oxidant enzymes, is mediated through transcription factor Nrf2 [AMERICAN JOURNAL OF PHYSIOLOGY; Alam,J; 284(4):F743-F752 (2003)]. The Carbon Monoxide (CO) produced by HO enzyme also protects against oxidative stress, at least partially by inhibiting proinflammatory cytokines [AMERICAN JOURNAL OF PHYSIOLOGY; Neto,JS; 287(5):F979-F989 (2004)].
The proinflammatory cytokine Tumor Necrosis Factor-alpha (TNF−α) can increase gene expression for synthesis of glutathione, whereas corticosteroids (which suppress both inflammation and immune response) reduce glutathione synthesis [JOURNAL OF BIOLOGICAL CHEMISTRY; Rahman,I; 274(8):5088-5096 (1999)]. The isothiocyanate sulforaphane found in broccoli is a very potent phase 2 enzyme inducer that raises cellular glutathione. Sulforaphane is a potent inducer of the enzyme heme oxygenase which catalyzes the formation of the bilirubin from heme (of hemoglobin) [FOOD AND CHEMICAL TOXICOLOGY; Fahey,JW; 37:973-979 (1999)].
Serum paranoxonase 1 has antioxidant actions on HDL and LDL cholesterol. Pomegranate juice polyphenols increase binding of paranoxonase 1 to HDL cholesterol [NUTRITION; Fuhrman,B; 26(4):359-366 (2010)], and may substantially reduce atherosclerosis [CLINICAL NUTRITION; Aviram,M; 23(3):423-433 (2004)].
(More detail about antioxidant enzymes can be found under The Free Radical Theory of Aging.)
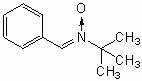
When chemists began their attempts to study free radicals, they found it difficult to measure steady-state concentrations because of the extremely short half-life of these chemical species. Molecules were discovered which could "trap" free radicals, and these molecules were appropriately called spin traps. When the free radical molecule comes in contact with the spin trap molecule, it sticks to the spin trap molecule, forming a larger, more stable molecule that can be measured by Electron Spin Resonance (ESR) spectroscopy.
|
The molecule alpha Phenyl t-Butyl Nitrone (PBN) has been one of the most successful spin-trapping agents used by chemists to identify free radicals such as the hydroxyl radical and the superoxide ion. Although spin-trapping agents are generally not defined to be antioxidants, their capacity to neutralize free radicals gives them the same functional capacity of antioxidants. Although PBN and some of the other "first generation" spin-trapping agents are toxic [FEBS LETTERS; Haseloff,RF; 418(1-2):73-75 (1997)], PBN may even be able to extend the maximum lifespan of mice by 5% when given at 24.5 months of age (from 31.7 months to 33.3 months) [BIOSCIENCE, BIOTECHNOLOGY, AND BIOCHEMISTRY; 62(4):792-794 (1998)].
Searches for more powerful, more stable and more water soluble spin-trapping agents have yielded such compounds as cyclic nitrone spin traps, which have 20-25 times the trapping capability of PBN [JOURNAL OF BIOLOGICAL CHEMISTRY; Thomas,CE; 271(6):3097-3104 (1996)]. The so-called "second generation" spin-trapping agent NXY−059 given 15 minutes after rats had been subjected to 2 hours of ischemia reduced infarct volume and neurological impairment by 59% [BRITISH JOURNAL OF PHARMACOLOGY; Sydserff,SG; 135(1):103-112 (2002)]. Monkeys subjected to experimental stroke showed more than 50% less brain damage with NXY−059 compared to saline [STROKE; Marshall,JWB; 32(1):190-198 (2001)]. These results are remarkable, given the fact that NXY−059 cannot cross a blood-brain barrier that has not been damaged by ischemia. Spin-trapping agents show promise of providing better protection against free radical damage than traditional antioxidants.
Anything that boosts the immune system is protective against cancer, but antioxidants have an additional anti-carcinogenic effect through protection against DNA damage. As much as 30% of cancer risk has been attributed to diet, mostly to the antioxidants in fruits and vegetables. Antioxidant supplements have much less frequently been credited with reducing cancer risk, but studies have been limited.
Free radical production has been shown to increase with age in studies of insects and mammals, and the rate of increase is in inverse proportion to the lifespan of the species [ANNALS OF THE NEW YORK ACADEMY OF SCIENCES; Sohal, RS; 663:74-84 (1992)]. CRAN (Caloric Restriction with Adequate Nutrition) reduces free radical production and extends maximum lifespan. But only a few animal experiments have indicated extension of maximum lifespan by antioxidant substances. Even if these experiments were valid, the results may have been due to effects other than antioxidant action. Many more studies and many larger studies on this subject would be greatly beneficial. "An ounce of prevention is worth a ton of cure" — reducing free radical production in the first place is far more efficient than trying to neutralize free radicals after they have been produced.
For details on many antioxidants which can be taken as supplements, see my Nutraceuticals Topic Index. For more about the molecular basis of the aging process, see my essay Mechanisms of Aging.