Issues concerning cooling impose constraints on the successful practice of cryonics. In an attempt to see these issues in perspective I have tried to see what can be learned from materials science, food science and cryobiology concerning properties that change with temperature — including such parameters as viscosity, thermal expansivity, specific heat capacity, thermal conductivity, compressibility, glass transition temperature, etc. I focus my attention on the cooling of a cryonics patient who is loaded or being loaded with vitrifying cryoprotectant, but I begin by discussing the classical cooling methods in cryobiology.
(For details on the initial cooldown immediately following deanimation, see Emergency Preparedness for a Local Cryonics Group.)
Cryonics is not cryobiology and cryobiology is not cryonics. Cryonics is the cryopreservation of humans & animals who are damaged by disease, aging and the cryopreservation process itself in the hope & expectation that future science will be able to repair that damage. Cryobiology is an established contemporary science that studies low temperature effects on organisms and attempts to apply that knowledge to cryopreservation. Cryonicists benefit by learning from cryobiologists.
Organ preservation has the best chance of succeeding by the use of vitrification, which is why cryonicists are so interested in that method of cryopreservation. But many cryobiologists continue to use classical cryopreservation methods that involve freezing because these methods minimize cryoprotectant toxicity and can be tolerably successful when applied to cells or embryos (rather than tissues or organs). Some of the principles underlying classical cryopreservation methods are relevant to vitrification, so it is useful for cryonicists to understand them.
The water inside living cells can be roughly divided between water that is osmotically active and water that is tightly bound to proteins, DNA & phospholipids — mainly proteins. Ions are also hydrated by water, which is why hydrated potassium (atomic number 19) ions (having a hydrated diameter of 3.8 Angstroms) can pass through smaller ion channels than sodium (atomic number 11) ions (which have a hydrated diameter of 5 Angstroms). If a cell is osmotically dehydrated by exposure to a hypertonic environment the amount of water that cannot be osmotically removed is about 10% of the total cell water. The fact that 10% of cell water is bound water (water "bound" by hydrogen bonding to macromolecules) has been confirmed by NMR, dialectric measurement, adsorption isotherms and calorimetry. Calorimetry demonstrates that bound water displays no heat of fusion — ie, the osmotically inactive water in a cell does not freeze. Bound water is so viscous that it vitrifies at cryogenic temperatures. Differential Scanning Calorimeter (DSC) measurements of cells subjected to cooling also indicate that about 10% of cell water is incapable of freezing [CRYOBIOLOGY; Schrenders,PD; 33(5):487-501 (1996) and CRYOBIOLOGY; Sun,WQ; 38(4):372-385 (1999)].

|
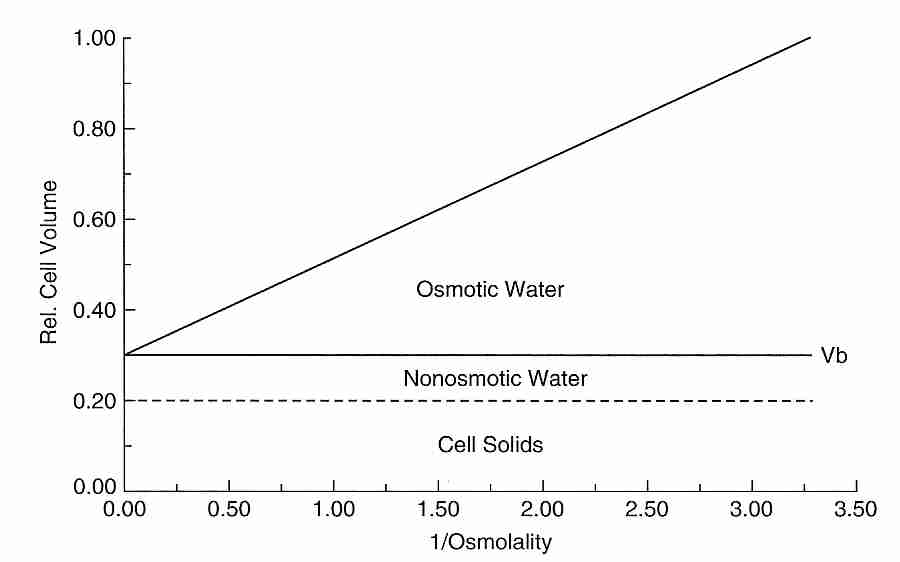
Cryobiologists often attempt to quantitatively determine the percent of freezable, osmotically active water in a cell and the percent of the cell volume which is osmotically inactive. The osmotically inactive portion of the cell consists of both cell solids and bound water. Cryobiologists typically estimate the osmotically inactive fraction of a cell by the use of a Boyle-van't Hoff plot, which graphs cell volume as the dependent variable and reciprocal of external osmolality as the independent variable. Osmolality is the count of osmotically active particles in a solution (ie, moles of solute per kilogram of solution for all solutes in the solution). The higher the osmolality of the external solution, the more water will osmotically leave the cell and the more the cell will shrink in size to a smaller cell volume. A cell can be exposed to several osmolalities and the resulting cell volumes can be measure. When these values are placed on a Boyle-van't Hoff plot they should lie approximately on a straight line (the Boyle-van't Hoff equation) because the relationship between cell volume and external osmolality is linear when osmolality is not too low. Extrapolating the line to the vertical axis gives the osmotically inactive cell volume, corresponding to an infinite external osmolality and a cell that has shrunken from the loss of all of its cell water. In the example shown, 30% of the cell volume is osmotically inactive — composed of 10% bound water (non-osmotic water) and 20% cell solids. A study of human oocytes using a Boyle-van't Hoff plot found that nearly 20% of the cell water is osmotically inactive [JOURNAL OF REPRODUCTION AND FERTILITY; Newton,H; 117(1):27-33 (1999)].
Cells may tolerate loss of osmotically active water, but removal of
bound water is extremely detrimental to viability. Without bound water,
proteins lose their structure and membranes fuse
together [CRYOBIOLOGY; Sun,WQ; 38(4):372-385 (1999)].
There is evidence, however, that reduction of cell volume by loss of osmotic
water beyond a critical level causes injury that cannot be attributed
to electrolyte damage [CRYOBIOLOGY; Williams,AJ; 17(6):530-539 (1980)].
|
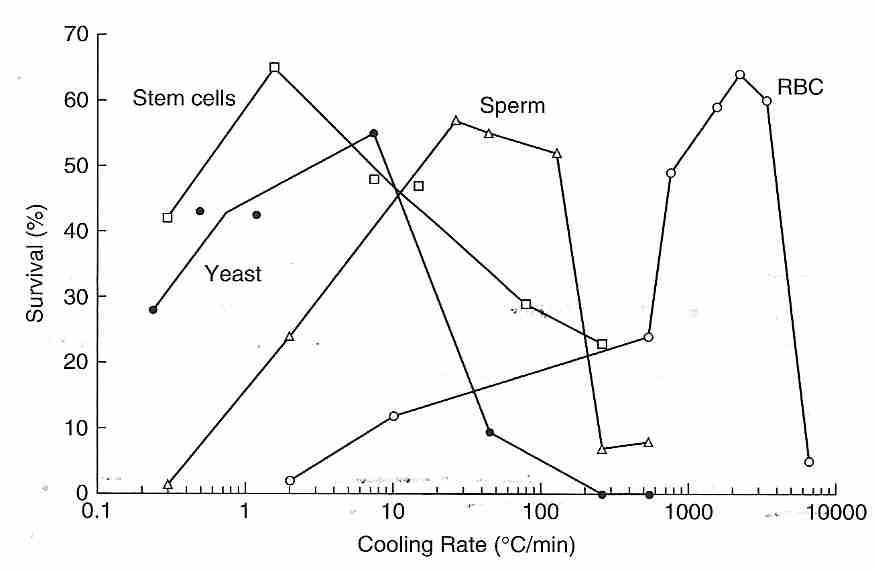
The classical (non-vitrification) cryopreservation methods of cryobiology are devoted to freezing cells or small collections of cells (embryos) at a cooling rate that minimizes loss of viability. The illustration indicates that roughly 60% of cells can survive with a cooling rate of 1ºC/minute (stem cells), 25ºC/minute (sperm) or 5000ºC/minute (RBC, Red Blood Cells). Ice forms much more readily (at higher temperatures) outside of cells than inside cells because nucleators are stronger and more plentiful outside of cells. The isotonic salt concentration in the human body is about 0.9% NaCl (sodium chlride), whereas the eutectic freezing temperature of a solution of NaCl is 22 wt%. Pure water ice forms in the extracellular space of tissues, leaving an increasingly concentrated salt solution — until the concentration reaches 22 wt%, at which time the eutectic mixture will freeze. The rising extracellular salt concentration in the unfrozen portion causes more water to osmotically leave the cells. When the cell has been dehydrated of all osmotically active water the remaining bound water and protein can vitrify the cell.
Cell survival generally drops to zero for cooling rates that are either too rapid or too slow, giving an "inverted U curve". Excessively fast cooling rates may kill cells by osmotic damage to membranes caused by pressure & friction from the exiting water molecules. Or excessively fast cooling allows for formation of ice inside cells — which is far more damaging than extracellular ice. There is evidence that plasma membrane damage due to excessive osmotic pressure creates holes in the membrane allowing ice to form intracellularly [BIOPHYSICAL JOURNAL; Muldrew,K; 66(2 Pt 1):532-541 (1994)]. With ultra-rapid cooling, however, water does not have time to leave cells, and ultra-rapid cooling may cause intracellular vitrification — both effects increasing cell viability [CRYOBIOLOGY; Dumont,F; 46(1):33-42 (2003)]. If cooling is too slow, however, cells will be killed either by prolonged exposure to the toxic concentrations of electrolytes that form outside the cell or by mechanical crushing from extracellular ice. Farrant's two-step method is to cool rapidly to a slightly subzero temperature, hold at that temperature until osmotic equilibrium is established, and then resume rapid cooling.
Optimum cooling rate is a function of membrane permeability to water and
the temperature dependence (activation energy) of membrane
permeability [ HUMAN REPRODUCTION; Devireddy,R; 15(5):1125-1135 (2000)].
The reason why red blood cells (RBCs) have such a high optimal cooling
rate for cell survival is because they have a high number of water channel
proteins (aquaporins) that allow for rapid water passage through
the cell membrane [JOURNAL OF BIOLOGICAL CHEMISTRY; Yang,B;
276(1):624-628 (2001)]. Stem cells, like most cells, do not have aquaporins
and so water crossing the cell membrane can only do so by diffusion through the
phospholipid bilayer.
|
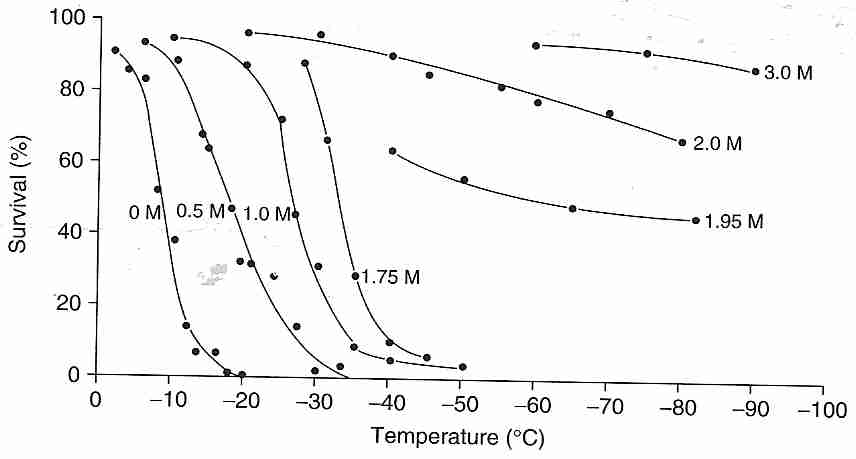
Cryoprotectants are generally used by cryobiologists to enhance the cryopreservation-by-freezing process rather than for vitrification. Cryobiologists seek to reduce (but not eliminate) ice formation while minimizing exposure to cryoprotectant toxicity. As shown in the illustration, glycerol added to human red blood cells moves the cell survival curve to lower temperatures — and even eliminates killing of all cells for concentrations of 1.95 Molar (moles per liter) and greater for slow cooling (1.7ºC/minute) and rapid thawing. The mere presence of ice in a solution can result in denaturing of protein that absorbs to the ice — a phenomenon that can be prevented with small amounts of cryoprotectant [BIOPHYSICAL JOURNAL; Strambini,GB; 70(2):971-976 (1996)]
Although cryoprotectants are present in concentrations insufficient to vitrify protect cells that are cooled slowly, they offer no protection to cells cooled at excessively high cooling rates because water has insufficient time to diffuse out of the cell and/or is damaging cell membranes by the velocity of the osmotic diffusion. Not only do cryoprotectants diffuse across cell membranes at different rates, cryoprotectants affect the speed with which water crosses membranes. For human sperm, water crosses cell membranes about three times faster with glycerol than with DMSO, and about three times faster with ethylene glycol than with glycerol at 22ºC [BIOLOGY OF REPRODUCTION; Gilmore,JA; 53(5):985-995 (1995)]. Both water and cryoprotectants cross cell membranes more slowly at lower temperatures.
Possibly the most common cryoprotectant and cryoprotectant concentration used by cryobiologists is 10% DMSO. Protocols using 10% DMSO have been adapted from bone marrow to umbilical cord blood stem cells [CRYOBIOLOGY; Hunt;CJ; 46(1):61-75 & 76-87 (2003)]. A common protocol is to cool at the optimum cooling rate for 10% DMSO between 0ºC and some plunge temperature between −30ºC and −40ºC, and then to plunge the sample into liquid nitrogen at the plunge temperature. With extracellular ice formation and diffusion of DMSO into the dehydrating cells, the concentration of DMSO in cells at plunge temperature could be around 40%.
Despite the fact that there has been some success in vitrification of animal organs, many cryobiologists are still struggling to cryopreserve single cells. Fish eggs (oocytes) are particularly challenging because of their high lipid content and large size. A large size makes cryoprotectant application more difficult because of the low surface area available for diffusion relative to cell volume. For a sphere, surface area S = 4πr2 and volume V = (4/3)πr3, which means that S/V varies inversely with r (radius). In other words, as the radius increases the amount of surface area relative to volume decreases.
Zebrafish embryos are also a favored candidate for cryopreservation by freezing methods. Zebrafish have become a model organism for studying vertebrate development and genetics. But the embryos have a low permeability to water and cryoprotectants, and the embryos have never been successfully cryopreserved. Freezing cryopreservation methods have successfully been applied to other marine embryos such as oysters and sea urchins.
(For a book that summarizes state-of-the-art cryobiology as of 2004, see LIFE IN THE FROZEN STATE.)
![[Specific volume changes at T<SUB>m</SUB> and T<SUB>g</SUB>]](./glassy.gif)
|
By the use of high cryoprotectant concentrations in biological cells, tissues and organs, solidification upon cooling to cryogenic temperature can be by vitrification to a glassy solid rather than by freezing — and there will be no ice crystal formation. The change from a syrupy liquid to a glassy solid that occurs at the glass transition temperature (Tg) can be quantified in terms of a sudden increase in viscosity. Viscosity at Tg is about 1012 Pascal-seconds or (equivalently) 1013Poise, since ten Poise equal one Pascal-second. [Poise as a unit of viscosity is a relic of the obsolete cgs system, equal to (dyne/cm2) X second, but it is still very widely used.] Tg is less precise than melting point temperature (Tm) insofar as an order-of-magnitude increase in cooling-rate can increase Tg by 3−5ºC. Slower cooling rates allow molecules time to minimize "free volume". Liquids classified as "strong glass formers" such as silica (SiO2) show only a small change in viscosity at Tg, whereas so-called "fragile" liquids such as polymers show very large changes in viscosity at Tg [NATURE 410:259-267 (8 March 2001)].
Two other variables which show distinctive changes at Tg are specific volume and specific heat capacity. Specific volume means volume per unit mass, ie, m3/kg in SI (and MKS) units. The word specific means "per unit of mass", as distinct from "per mole". Most materials experience decreasing specific volume with declining temperature. At Tm there is stark drop in specific volume followed by a distinct decrease in the rate of decline of specific volume with decline in temperature. By contrast, the region over which Tg occurs shows only a change in the rate of decline of specific volume with decline in temperature.
At Tg there is also a distinct decrease in the rate of decline of specific heat capacity with decline in temperature. Specific heat capacity refers to the amount of heat added or removed per unit mass to produce a unit change in temperature — in contrast to molar heat capacity which is the amount of heat per mole of material to produce a unit temperature change. (The term specific heat capacity is usually used in the abbreviated forms "specific heat" or "heat capacity". Specific energy capacity would be a more accurate term because gas temperature can be changed by compressive work, not just by heat.) Liquid water has a very high specific heat capacity compared to most other materials. At 4.2 kilojoules per kilogram-kelvin water has over ten times the specific heat capacity of copper. (The amount of heat required to warm water 1ºC at 14.5ºC was the original definition of calorie, but a calorie is now defined as 4.184 Joules.) (Glycerol has about half the specific heat capacity and half the thermal conductivity of water.)
The high specific heat capacity of earth's oceans prevents large temperature changes on our planet. The large water content of animal tissue is likewise protective against rapid temperature changes in warm or cold environments. The specific heat capacity of the solid (non-water) portion of fresh beef is 0.84 and — combined with the water portion — gives fresh beef a specific heat capacity of 3.14, which could be taken as a proxy for the specific heat capacity of a cryonics patient. The specific heat capacity of glycerol (and similar cryoprotectants) is about 2.4. A study of specific heat capacities and declining specific heat capacities of cryoprotectants and cryonics patients could be useful in designing scientific cooling equipment for use in cryonics. Changes in thermal conductivity of cryonics patients (produced with various cryoprotectants) with declining temperature should also be studied.
The specific heat capacity of all materials declines to a value of zero at a temperature of zero Kelvin — meaning that a tiny amount of heat could cause a huge temperature rise in any material near absolute zero. The change in the rate of change of specific heat capacity is so distinctive at Tg that it is used to determine Tg by scientists studying cryoprotectants. The instrument which measures this "thermal signature" is called a Differential Scanning Calorimeter (DSC). For most substances the change in rate of change of specific heat capacity at Tg increases with molecular weight — and Tg increases as molecular weight increases [PHARMACEUTICAL RESEARCH 11(1):54-59 (1994)].
[For further discussion of Tg see my essay Vitrification in Cryonics and Fragility thy name is glass]
Cryogenic temperatures can preserve mammalian tissues essentially unaltered for many centuries, if not many millenia [AMERICAN JOURNAL OF PHYSIOLOGY; Mazur,P; 247(3 Pt 1):C125-C142 (1984)]. Although vitrification above cryogenic temperatures results in a solid state, deteriorating chemical reactions can still occur in vitrified tissue at higher temperatures [PHARMACEUTICAL RESEARCH; Streefland,L; 15(6):843-849 (1998)].
How rapidly should a cryonics patient be cooled in the descent from 10ºC to −196ºC? To answer such a question scientifically it is helpful to study the properties of the materials concerned, namely water, cryoprotectant and biological tissue. Unpurified water, of course, has a freezing temperature close to 0ºC, but freezing is what cryonicists try to avoid or minimize. Instead, cryonicists are trying to vitrify as much as possible, ie, produce a glassy rather than a crystalline solidification in order to avoid mechanical damage by ice crystals. Water can be vitrified by cooling it at a rate of about 3 million ºC per second [JOURNAL OF MICROSCOPY 143(Pt 1):89-102 (1986)] to about −138ºC, but this cooling rate is impractical for cryonics. Fortunately, the rate of cooling required to produce a vitrified (glassy) solid rather than a crystalline solid is much less for solutions containing cryoprotectant than for pure water. The slowest rate of cooling which produces vitrification (prevents ice formation) is referred to as the critical cooling rate. (For more details on the process of vitrification, see my essays Vitrification in Cryonics and Lessons for Cryonics from Metallurgy and Ceramics.)
Like pure water, the cryoprotectant glycerol has a freezing temperature which is close to 0ºC, but mixtures of glycerol & water freeze at lower temperatures. A 50% w/w (weight/weight) solution of glycerol has a freezing/melting temperature (Tm) of −23ºC. The mixture with the lowest freezing temperature is roughly 66% glycerol, freezing at about −46ºC. But many cryonicists hope for something much better than frozen mixtures of glycerol and water. What is of more interest is the glass transition temperature (Tg, the temperature at which the cryoprotectant/water mixture hardens from a syrupy liquid to a solid glass). For glycerol mixtures Tg is close to −110ºC. The increasingly high viscosity of water/glycerol mixtures at lowering temperatures — and the absence of nucleation — means that the mixtures can be supercooled below the freezing/melting temperature without freezing. (Tg is typically about two-thirds the value of Tm on a Kelvin temperature scale for substances that vitrify.) With rapid cooling of glycerol/water mixture, ie, cooling at a rate in excess of the critical cooling rate, the mixture will vitrify at Tg rather than freeze at a higher temperature.
|
A 50% glycerol solution has a critical cooling rate of 100ºC per minute. By adding 6% w/w low molecular weight (400 gram/mole) PolyEthylene Glycol [PEG400] a critical cooling rate of 50ºC/min can be achieved for a 44% glycerol solution. Butane-2,3-Diol (2,3-BD), however, is a much more vitrifiable cryoprotectant than glycerol. A solution which is only 28.8% 2,3-BD has a critical cooling rate of 270ºC/min and addition of PEG400 lowers the rate to 20ºC/min [CRYOBIOLOGY 29(5):585-598 (1992)]. 2,3-BD is rather toxic, however, and for this reason researchers at 21st Century Medicine (21CM) have preferred using high concentrations of cryoprotectants with less glass-forming capability. Even cooling rates of 20ºC/min, however, have been far beyond the reach of cryonics practice.
More powerful vitrification solutions such as M22 and CI−VM−1 have allowed cryonicists to achieve vitrification at achieveable cooling rates. Similarly, cryobiologists have achieved vitrification of embryos and oocytes by reducing vitrifying cryoprotectant concentration (thereby reducing toxicity) and accelerating cooling rate. Smaller volumes of samples in cryoprotectant can be cooled at faster cooling rates than larger volumes [MOLECULAR AND CELLULAR ENDOCRINOLOGY; Arav,A; 187(1-2):77-81 (2002)].
When glycerol was used in cryonics it was desirable to cool as rapidly as possible to the freezing temperature of the estimated glycerol concentration (no lower than −46ºC). Because it is desirable for freezing to occur in the extracellular space rather than intracellularly (which is far more damaging), cooling below the estimated freezing temperature for glycerol concentrations used in cryonics should have been no faster than 1.0ºC/minute to allow time for the diffusion from the cells that accompanies extracellular freezing. Cooling at slower rates is a trade-off between cryoprotectant toxicity and risk of crystallization.
Although it has never been applied in cryonics or cryobiology, an oscillating electric field may reduce ice formation during rapid cooling. An oscillating electric field has been used to reduce the amount of ice formed when plunging 1.5 microliter samples of ethylene glycol (EG) into liquid nitrogen. Ice formation was reduced about 56% for a 3.5 Molar EG solution, and reduced about 66% for a 4.5 Molar EG solution [CRYOBIOLOGY; Jackson,JH; 34(4):363-372 (1997)].
When vitrifying cryoprotectants in cryonics patients (who always have nucleating agents in their bodies), however, cooling should be as rapid as possible to glass transition temperature (Tg) to prevent ice formation. The cooling rate would not need to be so fast for a pure vitrification solution, but perfusion cannot be perfect for cryonics patients and lower concentrations of vitrifying cryoprotectant will undoubtedly be present in many tissues. However, the exponential increase in cryoprotectant toxicity when cooling to Tg greatly reduces the danger of crystallization close to Tg [ANNALS OF BIOMEDICAL ENGINEERING; Rabin,Y; 33(9):1213-1228 (2005)].
Just below glass transition temperature cooling should quickly level-off to prevent cracking from thermal stress. Remaining just below Tg for a while can permit thermal stress to be relieved (annealing) prior to subsequent cooling. And subsequent cooling should be slow enough as to not cause cracking from thermal stress. Theoretically cracking could be prevented by slow-enough cooling, but because hydrogen bonds are easily broken a cooling rate to prevent cracking could take months — which is impractical. So cryonics patients are cooled to liquid nitrogen temperature over a period of about a week. The Cryonics Institute has computer controlled cooling boxes which allow pre-programming of the sequence of cooling steps desired in a cooling protocol.
The M22 vitrification solution from 21st Century Medicine [CRYOBIOLOGY 48:157-178 (2004)] has been demonstrated to tolerate supercooling without risk of ice formation for a 2 liter volume of pure cryoprotectant with a cooling rate of 0.15ºC per minute over 18 hours. Only at cooling rates of 0.10ºC per minute did a small number of ice crystals become visible. The critical warming rate of 0.4ºC per minute to prevent devitrification was sufficient for a 10mL volume of M22. (For details on the devitrification problem associated with critical warming rate, see The Devitrification Problem).
There are only two modes of heat transfer by which objects may be heated or cooled — conduction and radiation. Conduction is the transfer of heat energy from (or to) more energetic molecules to (or from) adjacent less energetic molecules by kinetic interaction (temperature is a measure of molecular kinetic energy). Radiation is energy transfer by means of electromagnetic waves (photons). Microwave radiation is a superior form of heat energy transfer in the sense that it can penetrate deep beneath the surface. Microwave cooling elevates temperatures uniformly rather than through conduction from the surface. I have long dreamed of an inverse form of microwave that could cool cryonics patients uniformly rather than through conduction at the surface.
In cryonics we cool patients with convection, a combination of conduction and fluid motion. In convection, a solid object (such as a cryonics patient) is cooled by a fluid (liquid or gas) that is rapidly circulated, such that the fluid can carry heat away from the conduction layer around the solid object. A person facing a cold wind will cool more quickly on their front than on their back because the convection of moving air adds to the conduction effect of contact with ambient temperature. In cooling a recently deanimated patient from human body temperature (37ºC) to 10ºC, cooling by rapid circulation of ice-water is far more effective than cooling by ice-packs or by standing water because of the convection effect.
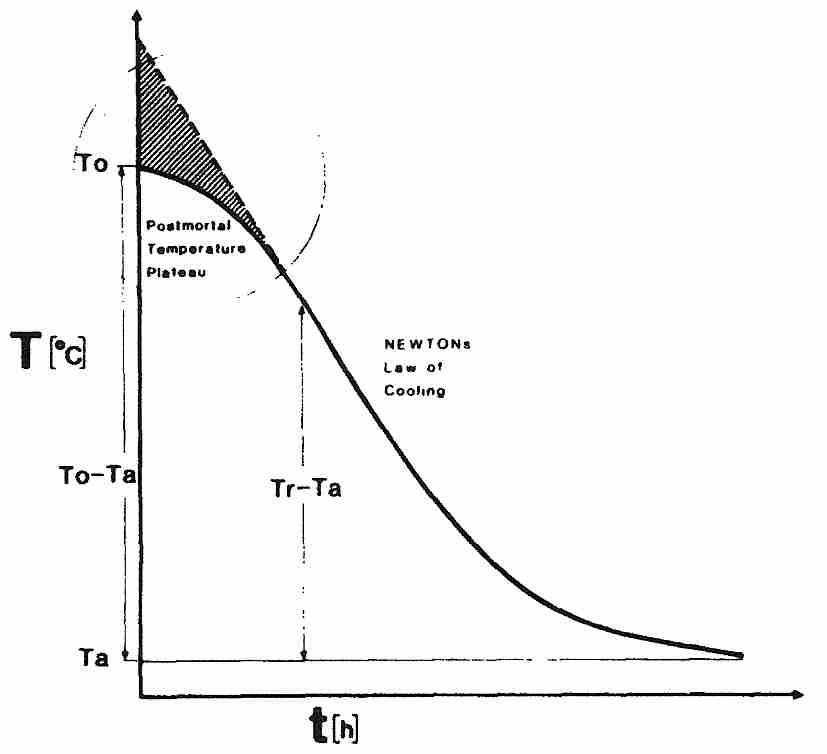
The formula governing convection is Newton's Law of Cooling which equates the rate of heat transfer to hA(Ts−Tf) where Ts is the starting temperature (head or body of a cryonics patient), Tf is the final temperature (temperature of the cooling medium, eg, ice water or cold nitrogen gas), A is the surface area of the solid and h is a variable which is dependent upon the rate of fluid motion as well as the thermal conductivity & heat capacity of the materials. Faster fluid motion and higher thermal conductivity will increase the value of h. In forensics it is a rule of thumb that a human body cools about twice as fast in still water — and about three times as fast in flowing water — as it would cool in air. This guideline does not specify water temperature or flow rate — which for cryonics should be ice water temperature and as fast a flow as is practical.
Thermal conductivity is a quantification of the ability of a material to conduct heat. Materials that readily conduct heat (such as metals) are distinguished from insulators with low thermal conductivity (such as foamed polystyrene, styrofoam). Water has a low thermal conductivity compared to metals, but has a high thermal conductivity compared to most organic solvents. The thermal conductivity of water at 25ºC (0.61 Watts per meter-Kelvin) is more than twice that of either glycerol or ethylene glycol (0.29 and 0.26 W/m−K, respectively) and is more than 20 times greater than nitrogen gas (0.026 W/m−K). Between 37ºC (body temperature) and 0ºC the thermal conductivity of water drops more that 10% (0.625 to 0.561 W/m−K).
Newton's Law of Cooling predicts that cooling rate is greatest at the start
of cooling when Ts is much greater than Tf, and the
cooling rate declines exponentially thereafter. This would be optimal for
cryonics purposes. Metabolic rate is halved for every 10ºC drop in temperature,
but the reduction of lipid peroxidation by cooling is greater than the reduction of
metabolic rate. Experiments on gerbils indicate that a drop in temperature from
37ºC to 31ºC nearly triples the amount of time that neurons can tolerate
ischemia [CRITICAL CARE
MEDICINE; Takeda,Y; 31(1):255-260 (2003)]. Rats subjected
to 2 hours of brain tissue ischemia showed a significant reduction in
neurological deficit as a result of only a 4ºC reduction of body temperature
for 5 hours, which began one hour following the start of
reperfusion [STROKE; Kollmar,R; 33(7):1899-1904 (2002)]. Pig eyeballs
show an exponential cooling curve in the first 13 hours after
death [EXPERIMENTAL PHYSIOLOGY; Kaliszan,M; 90(5):727-738 (2005)].
|
Newton's Law of Cooling, however, does not adequately describe the cooling of a cryonics patient, especially in the most critical initial stage immediately after legal death. Even though the heart has stopped beating, most body cells are still metabolically active and generating heat. The skin & subcutaneous fat have been designed to provide good insulation. Although the body is high in water content, body tissue has lower thermal conductivity than pure water. A human body which is initially at room temperature at the time of cardiac arrest — and which is subjected to no cooling medium other than room temperature air — will tend to remain at a normal body temperature plateau for a while before entering an exponential cooling curve toward room temperature. Someone lying on their back will expose 80% of their surface area to surface cooling, whereas in the foetal position only 60% of the surface is exposed. Although the cryonics patient is ideally in an ice bath immediately after legal death (and being metabolically supported by a heart-lung machine), thermal inertia due to heat-generating metabolism must be opposed.
Because cell metabolism (and cellular heat-generation) is highest immediately after legal death, the initial resistance to cooling results in a sigmoid-shaped curve — with the initial non-Newtonian plateau transitioning gradually into Newtonian form [FORENSIC SCIENCE INTERNATIONAL; Henssge,C; 38(3-4):209-236 (1988) and [FORENSIC SCIENCE INTERNATIONAL; Karhunen,PJ; 176(2-3):e17-e22 (2008)]. Fortunately, plateauing in the brain is only about one-third of that seen for the body in general [FORENSIC SCIENCE INTERNATIONAL; Al-Alousi,LM; 125(2-3):237-244 (2002)].
Newton's Law of Cooling is more directly applicable when cooling a cryonics patient after vitrification perfusion (typically from about 0ºC to glass transition temperature, Tg, which is roughly −125ºC for vitrifying cryoprotectants). There is little thermal inertia due to heat-generating metabolism at these temperatures. But attempting to speed cooling by lowering temperature (Tf) below Tg for a large vitrifiable specimen (cryonics patient) increases the likelihood of surface fractures [CRYOBIOLOGY 27(5):492-510 (1990)].
For many years Alcor cooled cryonics patients with a silicone oil ( poly dimethyl siloxane, also known as dimethicone) that remains fluid down to about −100ºC. This nontoxic material is similar to the silicone used in bathroom caulking and as a thickener in shampoos (add boric acid [H3BO3] and you get "silly putty"). Alcor used silicone oil bath to cool patients at a rate which would maintain a temperature difference of 10ºC between a temperature probe at the center of the patient's brain and the surface temperature — a cooling rate of about 0.1ºC/min. This rate of cooling may have relieved some of the stresses arising from freezing of glycerol/water mixtures. (Because silicone oil is so expensive, Alcor would recycle it by using plaster of Paris powder — calcium sulfate — to precipitate-out water and other contaminants.) With the use of superior cryoprotectant cocktails and ice blockers, it is believed that a negligible amount of ice is formed in Alcor's neuro patients, so the problem of stress-related cracking damage above Tg has been eliminated for neuros. More significantly, cooling can be done faster with vitrification because there is no longer a need to wait for osmosis to equalize the intra/extra-cellular cryoprotectant concentrations of freezing.
Now Alcor and the Cryonics Institute do convection cooling that cools the head to a temperature close to Tg at a rate of more than 0.4ºC/min using liquid nitrogen vapor. A high rate of gas flow can compensate for the low thermal conductivity & heat capacity of liquid nitrogen gas. As long as the vapor temperature is not below Tg there should be no solidification on the surface of the cryonics patient which could result in stresses and cracking. (For details on the cooling boxes used by the Cryonics Institute, see Computer-Controlled Cooling Boxes at CI.)
Although 21CM has discovered cryoprotectant mixtures that allow for vitrification with cooling rates of less than 1ºC/min cooling rates, no method of external cooling can cool the interior of a whole body cryonics patient at a rate much faster than about 0.2ºC/min — which precludes vitrification of whole body patients using existing cryoprotectant mixtures and external cooling. No method of external cooling can cool the interior of a head-only cryonics patient much faster than 1ºC/min, but this rate is just rapid enough to allow for vitrification.
Faster cooling rates could be achieved by the use of perflurocarbon, a substance with extremely low viscosity and which remains in a liquid state down to at least −100ºC. Rather than relying on the slow thermal conductivity from the surface of the cryonics patient, low temperature perflurocarbons can be perfused through the blood vessels. Mike Darwin did animal experiments with this procedure which achieved whole body cooling rates calculated to be 9ºC/min at 0ºC, falling to 1ºC/min at −90ºC. For brains he calculated his rates to be 50ºC/min at 0ºC falling to 5ºC/min at −90ºC. Cooling might be less than completely uniform, however, because once perflurocarbon displaces cryoprotectant fluid from some vessels it can circulate freely while leaving other vessels still clogged with cryoprotectant. As suggested in Patent 6,274,303 (which patents the use of perflurocarbon cooling for large organs and tissues), using a series of perflurocarbons of decreasing viscosity is a means to prevent this problem. The same patent asserts that vascular perfluorocarbon cooling can cool ten times faster than external cooling.
![[GRAPH COMPARING COOLING METHODS]](./external.gif)
|
The initial cooling of cryonics patients from human body temperature at the moment of legal death to around is extremely critical in reducing the amount of ischemic damage. Packing a cryonics patient in ice will result in a cooling rate that is no more than 0.064ºC/minute, which would take 6 to 8 hours to cool to 20ºC. Using a thumper and an ice bath can give cooling to 15ºC, and the addition of a squid can potentially cool to 10ºC in just over 2 hours (patient A-1049 was 2/3 the weight of the other two patients, which would result in faster cooling). Using cardiopulmonary bypass to cool a cryonics patient internally with a cold blood-washout solution can reduce cooling time to an hour, but a half-hour of surgery is required — and much ischemic damage happens during the half-hour of surgery. In 2011 Suspended Animation, Inc. announced that it was ready to deploy a lung lavage cooling system which had been developed for their use by Critical Care Research, a California company which is funded by the Life Extension Foundation. The lungs have a surface area about the size of a tennis court that is efficient for heat exchange as well as for gas exchange. Cold perfluorocarbon recirculated through the lungs can cool a cryonics patient at 1.2ºC/minute. Perflurocarbon lung lavage combined with a thumper and ice bath can cool a cryonics patient to 5ºC in 30 to 45 minutes without the delay of surgery associated with cardiopulmonary bypass cooling.
Something close to an effect similar to an inverse microwave might be possible by the use of adiabatic demagnetization. A cryonics patient could be perfused with a solution composed not only of cryoprotectant but of a paramagnetic salt such as ferric ammonium alum in a magnetic field. By gradually decreasing the strength of the magnetic field the salt ions become disordered, uniformly drawing heat throughout the body of the cryonics patient, not just from the surface. This or other novel mechanisms of cooling could potentially be of great benefit in solving the problem of rapidly cooling large biological objects.
It is possible that structural damage can be kept to a tolerable minimum even without full vitrification. Bull sperm is commercially cryopreserved by cooling at 15ºC/min — far less than the critical cooling rate — from 5ºC to −100ºC, followed by plunging in liquid nitrogen. Membranes are protected with the disaccharides sucrose & trehalose [CRYOBIOLOGY 37(3):219-230 (1998)]. All the sperm need not survive for the procedure to be practical when large amounts of sperm is used. In cryobiological applications where some freezing is tolerated, an inverted U-shaped survival curve is seen — with cells cooled either too slowly or too rapidly undergoing damage [FERTILITY AND STERILITY 60(5):911-918 (1993)].
Overly rapid freezing does not allow enough time for water to diffuse from the cells and freeze extracellularly — and it is intracellular freezing that causes the most damage. With faster freezing, however, the ice crystals that are formed are smaller and less damaging. The large crystals in foods that are frozen too slowly damage cell walls, leading to degredation of texture and loss of natural juices.
For years many cryonicists believed that 3.72 Molar glycerol (27.2%v/v) is optimal because it would allow 60% frozen water in the extracellular space while the intracellular water was vitrified. Light microscopy seemed to confirm this theory, but electron microscopy later refuted it. Thereafter, some cryonics organizations found better results by using the highest achieveable concentration of glycerol (just over 7 Molar). (Some cryonicists had formerly held the erroneous belief that overly high glycerol concentrations dissolve cell membranes.) Glycerol in water will vitrify at a concentration of 68%v/v (just over 9 Molar), but glycerol is so viscous that it is not possible to achieve concentrations much over 55%v/v (7.5 Molar) in a cryonics patient. So where glycerol is used as the cryoprotectant, cooling should be done at about 0.5ºC per minute to the solidification temperature and then at a rate of 0.1ºC to 0.2ºC per minute to lower temperatures. The faster the solidification temperature can be reached the less time there is for cryoprotectant toxicity and for ischemic damage (among other undesirable reactions) — and the greater the chance of supercooling to a lower solidification temperature. But if the cooling rate is faster than 0.5ºC per minute there may not be adequate time for cells to dehydrate, resulting in damaging intracellular freezing rather than freezing in the extracellular space.
Shipping of cryonics patients in liquid nitrogen is not feasible, but vitrification solutions used for cryonics are typically stable long enough that vitrified cryonics patients can be shipped at dry ice temperature. Dry ice is about 1.5 times more dense than water ice at dry ice sublimation temperature, but specific heat capacity of water ice is about twice that of dry ice. This consideration might suggest that it would be better to cool water ice to dry ice temperature for shipment. But this neglects the cooling effect of the latent heat of sublimation of dry ice. So shipment in dry ice is preferred. Shipping containers must allow for some venting of the sublimated carbon dioxide.
|
For vitrified patients below Tg and for non-vitrified patients loaded with cryoprotectant mixtures that have frozen solid at higher temperatures, problems with stress leading to cracking can become a significant concern. In materials mechanics stress quantifies force per unit area in a solid just as pressure quantifies force per unit area in a gas or liquid. Stress uses the same units as pressure: force per unit of area (newtons per square meter in SI units, called Pascals). Strain is a measure of the elongation of a solid material under stress. (Standing on a diving-board creates stress. The bending of the diving board is the strain.)
Material stiffness (Young's Modulus — called
modulus of elasticity, but better described as
modulus of inelasticity) is quantified as the ratio of
stress to strain. Strain can result not only from pressure stress, but
from thermal expansion stress. Coefficient of thermal expansion
is a property of materials, and for ice it is very high. The coefficient
of thermal expansion for ice is over five times greater than that of the
soda-lime glass of standard glassware, which means that ice can lead to
serious stresses. Thermal expansion stress is the
main reason ordinary glassware breaks so easily when heated. Replacing
some of the soda (Na2O) and lime (CaO) of soda-lime glass with
boron oxide (B2O3) results in pyrex, a glass
with one-third the thermal expansion of soda-lime — and far more resistant
to thermal shock. (Soda-lime glass has approximately the same specific
heat capacity as pyrex and 20% greater thermal conductivity — so
clearly it is the thermal expansion difference that contributes the
most to thermal shock resistance.)
|
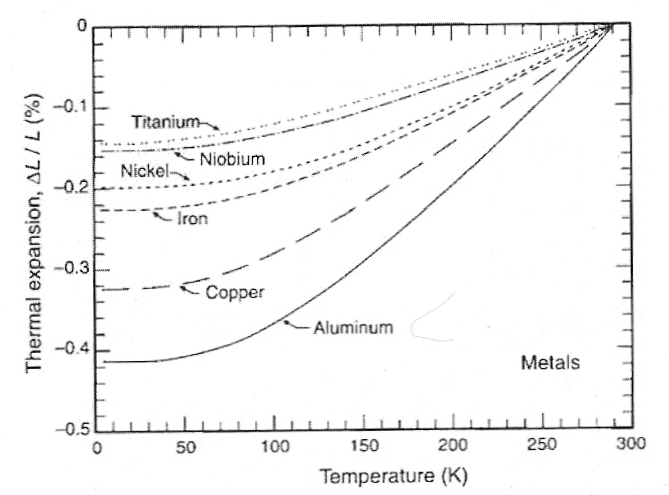
As temperature drops materials generally show a negative thermal expansion (i.e., a thermal contraction) in volume, which can be quantified along one dimension (linear thermal contraction). For example, compared to its length at ice water temperature (293 K = 0ºC), aluminum will contract 0.2% at 200 K (−73ºC) and 0.4% at 80 K (−193ºC, close to liquid nitrogen boiling temperature). By contrast, pyrex shows much less contraction: about 0.027% at 200 K (−73ºC) and 0.054% at 80 K (−193ºC). Teflon (which very strongly hydrogen bonds), on the other hand, shows considerably greater thermal contraction: 1.24% at 200 K (−73ºC) and 1.93% at 80 K (−193ºC). The hydrogen-bonding (and thermal contraction) of nylon is probably more comparable to what would be expected for biological tissues and cryoprotectants. (Data from Chapter 2 of HELIUM CRYOGENICS.)
The thermal conductivity at liquid nitrogen temperature is nearly four orders of magnitude greater for copper compared to non-crystalline, non-metallic materials like nylon, teflon, biological tissue, or cryoprotectants. Metals have high thermal conductivity due to electrons, whereas crystal thermal conductivity is due to phonons. Pure synthetic diamond has a thermal conductivity that is nearly five times greater than silver (despite the very low electrical conductivity of diamond) due to the lattice phonons. Water has a thermal conductivity that is nearly one thousandth that of silver, but water has about twice the thermal conductivity of glycerol, and about four times the thermal conductivity of rubber. Substances with low thermal conductivities are thermal insulators. Substances with low thermal conductivity (like substances with high thermal expansion) develop more thermal stress during cooling compared to substances with higher thermal conductivity.
Material resistance to cracking is also a function of the ductility or brittleness of the material. Ice & glasses are among the most brittle — least ductile — of materials. Ductile materials can endure much more plastic deformation prior to fracture than can brittle materials. Nearly all materials become less ductile — increasingly brittle — with declining temperature.
With low thermal conductivity and convection cooling at the surface of the cryonics patient, differences in thermal conduction between the surface and the interior can lead to thermal stress — and ultimately to cracking — if cooling rates are too rapid. Glasses have lower thermal conductivity than crystalline materials, which makes the problem serious for fully vitrified cryonics patients than for partially frozen cryonics patients, despite the lower thermal expansion of glasses.
|
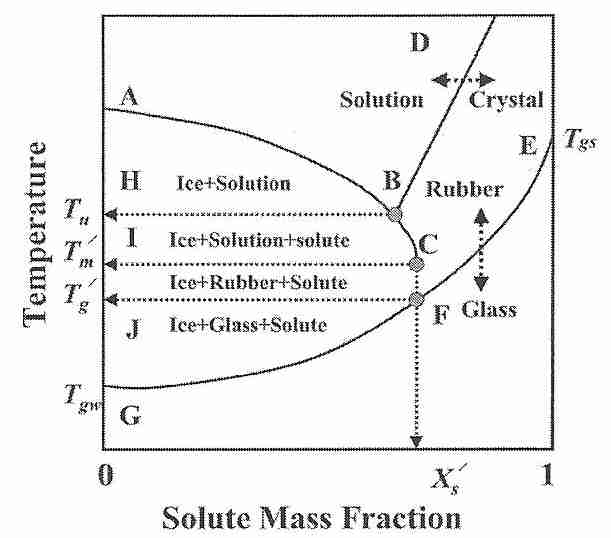
If cooled at a rate of about 3 million ºC per second, pure water will vitrify at a temperature of about −135ºC (Tgw). A cryoprotectant solution that will vitrify at a minimal concentration at a temperature Tgs will also vitrify at a higher concentration at a higher temperature (also called Tgs). If part of the vitrification solution freezes for some reason, the removal of ice (water) will mean that the remaining vitrification mixture is more concentrated. The lowest temperature at which freezing can occur is concentration dependent — a eutectic concentration will freeze at the eutectic temperature (Tu). [For further discussion of eutectic concentration, see Solidification of Eutectic Mixtures]. The lowest temperature at which freezing in an unfrozen portion (solution) can occur is the melting temperature Tm'.
The glass transition temperature of the concentrated unfrozen portion is referred to as Tg'. If ice is formed in a mixture intended to vitrify — as in an imperfectly perfused cryonics patient — the vitrification mixture remaining in the unfrozen portion will have a higher concentration and, thus, a higher Tg (which will be Tg') [Figure 1; CRYOBIOLOGY; Wowk,B; 60(1):11-22 (2010)]. 57(1):43-48 (2009)]. A practical significance of this fact is that if a cryonics patient is partially vitrified at a Tg' that is significantly above the expected Tgs, cracking due to thermal stress can occur if cooling is not slowed at that higher Tg' and there is no change in thermal expansivity. [For further discussion of Tg', see Some Properties of Glass.]
Borax can raise Tg' by cross-linking with solutes and thereby increasing effective molecular weight [CHEMICAL & PHARMACEUTICAL BULLETIN; Izutsu,K; 51(6):663-666 (2003)]. If borax can similarly raise Tgs and reduce thermal expansivity of vitrified specimens, it could reduce low temperature cracking. But would the cross-linking compromise critical structural information more than the cracking it reduced?
Pilot studies done on tissues at cryogenic temperatures (without cryoprotectant) showed kidney tissue to have more than twice the elasticity modulus and four-times the strength of brain tissue [CRYOBIOLOGY 33(4):472-482 (1996) and CRYOBIOLOGY 34(4):394-405 (1997)]. Another pilot study showed reduction of thermal expansion with glycerol & DMSO [JOURNAL OF BIOMECHANICAL ENGINEERING 120(2):259-266 (1998)].
Cooling of cryonics patients to liquid nitrogen temperature is done in a computer-controlled cooling box. Temperature probes (thermocouples) are used to monitor the temperature of the gas in the cooling box, in the chest of the patient, under the skin of the skull, or deep in the nasopharynx (ie, deep down the nose into the upper throat). A probe in the chest gives core body temperature whereas the nasopharyngeal probe gives a good representation of core brain temperature. (We do not want to damage the brain by driving a probe into the center of the brain.)
One thermocouple will control cooling rate by controlling the rate & duration of opening of the valve which releases liquid nitrogen into the cooling box. The other thermocouples simply monitor temperature. Vaporization of liquid nitrogen is an endothermic (heat-absorbing, entropy-driven) process which contributes significantly to the cooling effect.
For good vitrification it is important to cool as quickly as possible to Tg (assumed to be around −123ºC) after perfusion is complete. Rapid cooling reduces exposure time to cryoprotectant toxicity and it ensures a rate of cooling above the critical cooling rate — supercooling the vitrified patient and avoiding devitrification.
In the initial cooling phase, the liquid nitrogen control valve on the cooling box will essentially be open all the time no matter which thermocouple is used as the controlling thermocouple. But as Tg is approached, deep thermocouples can cause serious overshooting to excessively low temperature for less deep tissues. Core brain and (especially) core body temperature drops much more slowly than superficial temperatures due to the conduction time required for the lower temperature to go from the surface to the core. For this reason, if a core temperature (or one closer to the core, such as neck/lower throat) is used for the controlling thermocouple, the more superficial tissues will cool excessively. This is particularly true initially when cooling is most rapid. On the other hand, if ambient gas temperature is used to control the valve that sprays liquid nitrogen into the cooling box, there can be excessive thrashing of the temperature, with frequent opening and closing of the valve.
The controlling thermocouple should cool to just above Tg. Although the rate of nucleation is very high just above Tg, the rate of crystal growth is very low. So it is safe to hold a vitrified cryonics patient just above Tg, when patient perfusion is thorough. (For patients who have perfused poorly due to ischemia, edema or stroke/atherosclerosis it may be best to go rapidly below Tg and risk increased cracking than to remain near Tg and risk freezing.) It is important that there be minimal temperature gradient in the patient's head when cooling below Tg because lack of uniform temperature is the greatest cause of thermal stress leading to cracking below Tg.
Tg for cryoprotectants used in cryonics is about −123ºC. Although solidification (glassification) of a vitrified human brain does not occur until temperature drops below −123ºC, the viscosity is so high at higher temperatures that a great deal of stress relief can be accomplished by holding at temperatures well above Tg. Thus, there can be considerable stress relief by holding a patient with a vitrified brain at −100ºC, at −110ºC, and at −120ºC for 24 hour periods. Reportedly, a patient held at −110ºC for three months did not exhibit cracking until nearly −140ºC, whereas a patient held at −110ºC for only one month cracked at a much higher temperature.
Following the last of the three holding steps, at −120ºC, the human could then be warmed to −118ºC in 30 minutes and then held at −118ºC for 2 hours as a means of annealing — not only increasing stress relief through increased diffusion at higher temperature, but allowing the surface temperature to come closer to the core temperature (greater temperature uniformity results in less thermal stress).
Experiments with vitrifiable concentrations of cryoprotectant propylene glycol (Tg, −108ºC) have demonstrated that even very slow and uniform cooling of a 482-ml sample cannot prevent fracturing for temperatures lower than 25ºC below Tg (−132ºC) [CRYOBIOLOGY; Fahy,GM; 27(5):492-510 (1990)]. and there would surely be considerably more damage approaching liquid nitrogen temperature (−196ºC). Nonetheless, both a rabbit kidney and a rabbit hemi-brain have been cooled to liquid nitrogen temperature without cracking using newer vitrification solutions. This result cannot be expected from larger samples. Larger volume samples fracture at higher temperatures than small volume samples when the cooling rate is the same [Table 2; CRYOBIOLOGY; Fahy,GM; 27(5):492-510 (1990)].
Every metal has a cooling rate above which stresses leading to cracking can occur, whereas slower cooling rates do not lead to cracking. There is no reason why glass or ceramic materials need be different, and vitrified organs are reasonably uniform. The critical difference lies in the fact that metals and even non-biological glasses cohere by means of stronger (usually covalent) bonds than are found in the glasses formed by biologically vitrifying cryoprotectants. Hydrogen bonds are more easily broken, which means that to avoid thermal stresses leading to cracking in a cryonics patient being cooled to liquid nitrogen temperature might require cooling at rates in the order of a few thousandths of a degree per hour — not a cooling rate that can be achieved by any practical means.
Because cracking is inevitable in cooling a vitrified human brain to liquid nitrogen temperature, it is best to induce cracking at higher temperatures. Cracks induced at higher temperatures are fewer and broader, whereas if cracking does not occur until lower temperatures the resulting cracks will be extremely small and numerous [Figure 8; CRYOBIOLOGY; Fahy,GM; 27(5):492-510 (1990)] — which is more damaging. Therefore, the next step in the cooling protocol is to cool somewhat quickly (about 1ºC per minute) to −145ºC to induce higher temperature cracking.
Fracturing at higher temperatures reduces, but does not eliminate the possibility of fracturing at lower temperatures. Following the crack-inducing step, exponential rather than linear cooling to −196ºC is the best means to reduce cracking due to thermal stress. Thermal expansivity declines as temperature declines (a precipitous decline of thermal expansivity occurs at Tg), but relaxation time (time for the stresses to be mostly relieved) increases even more than thermal expansivity decreases as temperature declines below Tg. Moreover, thermal conductivity declines with decreasing temperature (which is already very low for non-crystalline, non-metallic materials). As a result, thermal stress increases at an increasingly higher rate for each degree of cooling below Tg. Therefore, decreasing the rate of cooling as temperature drops results in less thermal stress than cooling at a constant rate below Tg.
The entire human cooling protocol — including exponential cooling below −145ºC — could be something like:
cool as fast as possible to −100ºC
hold at −100ºC for 24 hours (stress relief)
cool from −100ºC to −110ºC in 1 hour (10ºC/hour)
hold at −110ºC for 24 hours (stress relief)
cool from −110ºC to −120ºC in 1 hour (10ºC/hour)
hold at −120ºC for 24 hours (stress relief)
warm from −120ºC to −118ºC in 1 hour (annealing)
hold at −118ºC for 2 hours (annealing)
cool from −118ºC to −145ºC in 30 minutes (rapid-cooling cracking step)
cool from −145ºC to −170ºC in 25 hours (1ºC/hour)
cool from −170ºC to −190ºC in 50 hours (0.4ºC/hour)
cool from −190ºC to −196ºC in 24 hours (0.25ºC/hour)
For cryonics patients who are not vitrified, but for whom cryoprotectant/water mixtures freeze temperatures considerably above Tg, there will be considerable internal stress well above Tg. More rapid cooling of these patients may reduce the amount of freezing and the size of the ice crystals, but increase the amount of intracellular ice and the size of the cracks. Although cooling slow enough could theoretically eliminiate cracking, even with very slow cooling the risk of cracking becomes significant around −20ºC below Tg. (For either freezing or vitrification, the amount of internal stress created will increase with the size of the cryonics patient.) Future molecular technology will be required to repair both the cracking and the freezing — and it is difficult to know what mixture of cracking & freezing will be optimal for a technology which is still speculative. Without vitrification, storage at liquid nitrogen temperature seems most prudent, because that temperature will allow for the best preservation for the long wait for the required technology to develop. Liquid nitrogen temperature storage would be less vulnerable to radiation damage than higher cryogenic temperature storage. (See my essay Radiation Damage at Cryogenic Temperature.)
Based on the assumption that long-term storage of vitrified cryonics patients may necessarily need to be near Tg to avoid cracking or fracturing, engineers with the Timeship project have designed cryostorage units which can cheaply and robustly maintain the higher temperatures. When and if Timeship storage becomes available it is anticipated that it will be open to members of any cryonics organization. The cryonics organization Alcor has intermediate cryogenic temperature storage available for some neuro patients, but at a much higher cost than for liquid nitrogen storage.
(For some speculations about molecular mobility at cryogenic temperatures, see my essay Molecular Mobility at Low Temperature.)