by Ben Best
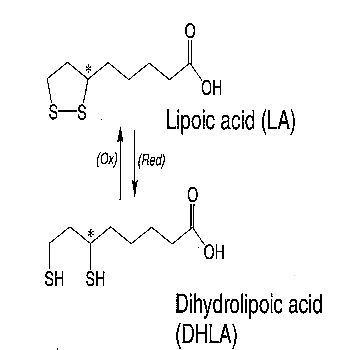
Lipoic Acid (1,2-dithione-3-pentanoic acid) is a sulfur-containing anti-oxidant with metal-chelating and anti-glycation capabilities. Unlike many anti-oxidants which are active only in lipid (fat) or aqueous (water) phase, lipoic acid is active in both lipid and aqueous phases.
Lipoic acid has been given for mushroom poisoning, heavy metal intoxication and diabetic neuropathy. By protecting against the lipid peroxidation aldehyde 4−HydroxyNonEnal (4−HNE), lipoic acid reduces glycation [ FREE RADICAL BIOLOGY & MEDICINE; Abdul,HM; 42(3):371-384 (2007)]. The anti-glycation capacity of lipoic acid combined with its capacity for hydrophobic binding enables lipoic acid to prevent glycosylation of albumin in the bloodstream. Lipoic acid is known to biochemists as being part of a prosthetic group (lipoamide) of the dihydrolipoamide acetyltransferase portion of the pyruvate dehydrogenase enzyme complex that converts pyruvate to Acetyl-CoA prior to entry into the citric acid cycle.

|
As with the thiol anti-oxidant glutathione, Lipoic Acid (LA) is part of a redox pair, being the oxidized partner of the reduced form DiHydroLipoic Acid (DHLA). LA is readily absorbed from diet (being particularly high in spinach) and is rapidly converted to DHLA by NADH or NADPH in most tissues. Unlike glutathione, for which only the reduced form is an anti-oxidant, both the oxidized and reduced forms of lipoic acid are anti-oxidants. LA is active against hydroxyl radicals, hypochlorous acid and singlet oxygen, but not against hydrogen peroxide or superoxide. DHLA is active against hydroxyl radicals and hypochlorous acid, but not against hydrogen peroxide or singlet oxygen. In general, DHLA has superior anti-oxidant activity to LA. DHLA can regenerate Vitamin C and Vitamin E from their oxidized forms [GENERAL PHARMACOLOGY 29(3):315-331 (1997)]. Although reduced glutathione (GSH) has twice the chemical reactivity in its thiol group, DHLA is superior to GSH in regenerating Vitamin C [BIOFACTORS; Bast, A; 17:207-213 (2003)]. By donating two hydrogens, DHLA can neutralize free radicals without itself becoming a free radical.
Due to an assymetric carbon having four different attached groups, lipoic acid exists as two enantiomers (mirror images which are chemically unique): the R-enantiomer and the S-enantiomer. Naturally-occurring lipoic acid is the R-form, but synthetic lipoic acid (known as alpha lipoic acid, α−lipoic acid) is a racemic mixture of R-form and S-form. Although the R-enantiomer is more biologically active than the S-enantiomer, administration of alpha lipoic acid actually results in greater formation of DHLA due to a synergistic effect which each enantiomer exerts on the reduction of the other [BIOFACTORS; Bast, A; 17:207-213 (2003)].
|
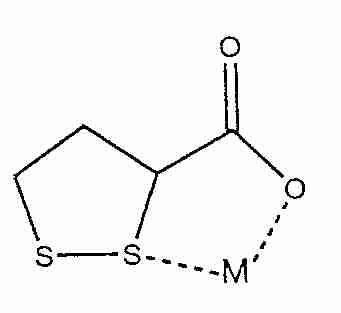
Both LA and DHLA can chelate heavy metals, but the R-form is more effective for chelation than alpha-lipoic acid [BIOCHEMICAL PHARMACOLOGY; Ou,P; 50(1):123-126 (1995)]. LA is most effective in chelating Cu2+, Zn2+ and Pb2+, but cannot chelate Fe3+. DHLA forms complexes with Cu2+, Zn2+, Pb2+, Hg2+ ( mercury) and Fe3+ that are poorly soluble in water. Although DHLA chelates Fe3+, it can also reduce Fe3+ to Fe2+ -- a pro-oxidant effect it shares with ascorbic acid. Insofar as most iron is tightly bound to ferritin protein, ascorbate reduction of Fe3+ rarely occurs, but DHLA may have the capacity to remove bound iron from ferritin. Nonetheless, LA fed to old rats reduced the increase of iron in the cerebral cortex associated with aging, without (apparently) affecting normal protein-bound iron — and by an unknown mechanism other than chelation [REDOX REPORT; Suh,JH; 10(1):52-60 (2005)].
Even small amounts of cadmium (Cd2+) can cause significant lipid peroxidation in the brain, which can be prevented by lipoic acid [FREE RADICAL BIOLOGY & MEDICINE; Packer,L; 22(1/2):359-378 (1997)]. Lipoic acid (DHLA) chelation of iron & copper in the brain may reduce free-radical damage contributing to Alzheimer's Disease [NEUROBIOLOGY OF AGING 23:1031-1038 (2002)]. Lipoic acid has been shown to protect against age-related increase in InterLeukin-1 (IL-1)ß: concentration possibly related to an age-related decline in arachidonic acid in the hippocampus causing impaired LTP and glutamate release [NEUROBIOLOGY OF AGING; McGahon,BM; 20:655-664 (1999)].
The regulatory gene sequence for producing antioxidant enzymes, both constitutively and by induction, is called the Antioxidant Response Element (ARE). Lipoic acid has been shown to restore ARE transcription activity in the liver of aging rats [PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES (USA); Suh,JH; 101(10):3381-3386 (2004)]. The anti-oxidant enzyme induction is evidently specific to the R-form of LA [REDOX REPORT; Lal,A; 13(1):2-10 (2008)]. Healthy young men given a three-day antioxidant treatment associated with intensity-resistance exercise showed a 16% reduction in lipid peroxidation (TBARS assay) with 1.2 grams/day α−lipoic acid.
The transcription factor NF−κB, which regulated many inflammatory cytokines, increases with age, leading to chronic inflammatory conditions. Lipoid acid has been shown to reduce NF−κB activity in a dose-dependent manner in human monocytic cell lines [EXPERIMENTAL GERONTOLOGY; Lee,HA; 37(2-3):401-410 (2002)].
Lipoic acid is also beneficial in reducing ischemic-reperfusion injury by direct action as well as by glutathione protection and xanthine oxidase inhibition [FREE RADICAL BIOLOGY & MEDICINE; Packer, L.; 19(2):227-250 (1995)]. Protection against peroxynitrite damage by lipoic acid is highly dependent upon the target molecule [JOURNAL OF BIOLOGICAL CHEMISTRY; Rezk,BM; 279(11):9693-9697 (2004)]. Protection of neurons from glutamate excitotoxicity is equally effective by the R-form and S-form [FREE RADICAL BIOLOGY & MEDICINE; Tirash,O; 26(11/12):1415-1426 (1999)].
In mitochondria lipoic acid can compensate for the low concentrations of glutathione in that organelle, and can chelate heavy metal ions that could generate free radicals. In old rats supplemented with R-lipoic acid, mitochondrial membrane potentials and oxygen consumption have been restored significantly while at the same time MDA (MalonDiAldehyde, a product of lipid peroxidation) was reduced to one-fifth of the un-supplemented level [FASEB JOURNAL; Hagen,TM; 13(2):411-418 (1999)]. Age-related damage to heart muscle cell mitochondria has been considerably reduced by lipoic acid supplementation [FASEB JOURNAL; Suh,JH; 15(3):700-706 (2001)]. Glutathione synthesis declines considerably with age, but lipoic acid has also been shown to restore glutathione synthesis to more youthful levels in aging rat liver [PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES (USA); Suh,JH; 101(10):3381-3386 (2004)].
Supplementation with both lipoid acid (LA) and acetyl-l-carnitine (ALCAR) is an effective way of improving mitochondrial metabolic function without increasing oxidative stress [PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES (USA); Hagen, T; 99(4):1870-1875 (2002)]. ALCAR supplementation in combination with lipoic acid substantially restored spatial memory capacity in experimental rats [PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES (USA); Liu,J; 99(4):2356-2361 (2002)]. The ALCAR/LA combination has been shown to partially or completely restore brain mitochondrial function of old rats to the level of young rats [NEUROCHEMICAL RESEARCH; Long,J; 34(4):755-763 (2009)]. The ALCAR/LA combination has significantly improved complex object discrimination and spatial learning in aged beagle dogs [THE FASEB JOURNAL; Milgram,NW; 21(13):3756-3762 (2007)].
Lipoic acid can be obtained in the diet from foods containing high metabolic activity. Meat from heart can contain ten times the amount of lipoic acid as meat from muscle. Spinach is also rich in lipoic acid. Lipoic acid is readily digested, absorbed and transported to tissues. Lipoic acid induces cystine/cysteine uptake, thereby increasing synthesis of glutathione.